2 江苏省南通市第一人民医院重症医学科 226001
2 Department of Critical Medicine, First People's Hospital of Nantong City, Nantong 226001, China
感染性休克病死率高、并发症多且治疗花费高[1-4]。液体复苏补充有效循环血量,从而改善组织灌注、纠正细胞缺氧,是感染性休克患者救治的重要环节。但是,研究显示在重症监护病房仅有约50%的感染性休克患者有容量反应性[5]。因此,更好地预测容量反应性对感染性休克的救治具有重要意义[6]。基于心肺交互作用机制的监测技术,将循环系统受呼吸运动影响的程度作为衡量指标,评估循环系统对液体负荷的反应性,具有无创、不额外增加液体负荷且可反复进行的特点,近年来逐渐受到关注[7-8]。本研究拟评估PEEP抬高试验[9]、呼气末屏气试验(EEO)[10]、每搏量变异率(SVV)及脉压变异率(PPV)等基于心肺交互机制的监测技术评价对感染性休克患者容量反应性的预测价值。
1 资料与方法 1.1 一般资料纳入2016年1月至2017年6月南通市第一人民医院重症医学科(ICU)收治的感染性休克患者,入选标准:①≥18岁;②符合2001年SCCM/ESICM/ACCP/ATS/SIS严重脓毒症及脓毒症感染性休克诊断标准[11];③窦性心律。排除标准:①妊娠;②颅内高压、肺动脉高压;③存在脉搏指示连续心输出量(PiCCO)监测禁忌。本研究方案经医院伦理委员会批准,所有治疗和监测的实施均得到患者家属知情同意。
1.2 研究方法所有入选患者均置入锁骨下或颈内静脉中心静脉导管(ARROW公司,美国)、经股动脉穿刺置入动脉导管(PV2014L16N,Pulsion Medical Systems,德国),通过IntellVue MP60监测仪(Philips公司,荷兰)持续监测心率(HR)、中心静脉压(CVP)、收缩压(SBP)、平均动脉压(MAP)及心脏指数(CI),记录试验前每搏量变异率(SVV)、脉压变异率(PPV)。各参数计算试验前后变化Δ参数(%)=(参数试验后﹣参数试验前)÷参数试验前×100%。采用容量控制通气模式,潮气量(VT)8~10 mL/kg,呼吸频率(RR)为12~16次/min。给予充分镇痛(布托啡诺)、镇静(右美托咪定、咪达唑仑)暂时抑制受试者的自主呼吸。试验期间患者体位、呼吸机参数及血管活性药物用量保持不变。
试验步骤如下:①PEEP抬高试验。在原有的PEEP水平上增加10 cmH2O(1 cmH2O=0.098 kPa),维持60 s,记录试验前后血流动力学参数;恢复PEEP至原先水平5 min;②呼气末屏气试验。呼气末屏气15 s,记录试验前后血流动力学参数;恢复原先机械通气5 min;③容量负荷试验。30 min内静脉输注复方氯化钠500 mL,记录输注前后CI。研究过程中患者平卧位、血管活性药物剂量均维持不变。容量负荷试验后CI增加(ΔCI)15%为容量反应阳性组,ΔCI < 15%为容量反应阴性组。如各试验阶段生命体征异常波动、呼气末屏气不能完成及容量负荷试验出现容量过负荷表现时立即停止试验,并剔除出本研究。
1.3 统计学方法采用SPSS 19.0进行统计分析,符合正态分布的计量数据以均数±标准差(Mean±SD)表示,两组间比较采用LSD-t检验,试验前后比较采用自身配对t检验;计数资料比较采用χ2检验或Fisher确切概率法;以受试者工作特征(ROC)曲线计算各参数的曲线下面积,采用最大Youden指数法取截断值,计算灵敏度和特异度;以P < 0.05为差异有统计学意义。
2 结果 2.1 一般资料入选感染性休克患者45例,其中肺部感染22例、肠道感染7例、腹腔感染6例、胰腺炎6例、其他感染4例。容量反应阳性患者占53.3%(24/45)。容量反应阳性组与容量反应阴性组患者性别、年龄、APACHEⅡ评分、机械通气平台压力(Pplat)、呼气末正压(PEEP)、中心静脉压(CVP)及心脏指数(CI)等指标比较差异无统计学意义(均P > 0.05),具有可比性,见表 1。
| 指标 | 容量反应阳性组(n=24) | 容量反应阴性组(n=21) | χ2或t值 | P值 |
| 性别(男/女) | 16/8 | 14/7 | 0.20 | 0.66 |
| 年龄(岁) | 69.5±16.3 | 71.0±17.1 | 0.30 | 0.77 |
| APACHEⅡ评分 | 20.5±5.9 | 19.3±6.5 | 0.63 | 0.53 |
| Pplat(cmH2O) | 20.3±3.9 | 22.0±4.8 | -1.28 | 0.21 |
| PEEP(cmH2O) | 5.6±2.1 | 6.0±2.3 | 0.63 | 0.53 |
| CVP(mmHg) | 9.0±3.8 | 9.1±4.3 | -0.15 | 0.88 |
| CI [L/(min·m2)] | 2.7±0.7 | 2.8±0.8 | -0.20 | 0.85 |
| 注:1 mmHg=0.133 kPa | ||||
如表 2所示,PEEP抬高试验后两组患者CVP显著升高(P < 0.05),SBP、MAP及CI显著降低(P < 0.05),ΔCVP、ΔMAP组间差异无统计学意义(P > 0.05),容量反应阳性组SBP、CI降低程度显著大于容量反应阴性组[ΔSBP:(9.2±5.6)% vs(6.6±4.8)%,P < 0.05;ΔCI:(11.9±7.0)% vs(5.8±4.4)%,P < 0.05]。呼气末屏气试验后两组患者CVP显著降低(P < 0.05),SBP、MAP及CI显著升高(P < 0.05),ΔCVP、ΔSBP组间差异无统计学意义(P > 0.05),容量反应阳性组MAP、CI升高程度显著大于容量反应阴性组[ΔMAP:(7.5±5.4)% vs(3.3±5.3)%,P < 0.05;ΔCI:(11.6±5.2)% vs(4.0±6.8)%,P < 0.05]。
| 指标 | PEEP抬高试验前 | PEEP抬高试验后 | PEEP抬高试验变化(%) | 呼气末试验前 | 呼气末试验后 | 呼气末试验变化(%) |
| HR(次/min) | ||||||
| 容量反应阳性组(n=24) | 110.6±13.0 | 114.5±11.0a | 4.0±7.4 | 109.9±12.8 | 107.8±9.3 | 1.5±6.4 |
| 容量反应阴性组(n=21) | 108.3±14.1 | 108.0±10.7 | 0.2±6.6 | 109.2±13.4 | 105.4±13.1a | 3.4±4.6 |
| SBP(mmHg) | ||||||
| 容量反应阳性组(n=24) | 111.5±10.9 | 100.4±9.1a | 9.8±4.6b | 111.5±11.4 | 120.3±10.3a | 8.1±6.5 |
| 容量反应阴性组(n=21) | 109.1±12.1 | 101.4±9.0a | 6.6±4.8 | 109.3±12.6 | 114.1±11.6a | 4.7±5.8 |
| MAP(mmHg) | ||||||
| 容量反应阳性组(n=24) | 73.3±11.0 | 66.4±9.0a | 9.2±5.6 | 73.8±10.5 | 79.1±10.2a | 7.5±5.4b |
| 容量反应阴性组(n=21) | 72.1±12.6 | 67.0±10.3a | 6.7±4.6 | 73.5±11.9 | 75.6±11.3a | 3.3±5.3 |
| CVP(mmHg) | ||||||
| 容量反应阳性组(n=24) | 9.3±4.1 | 10.1±4.2a | 12.6±12.4 | 9.2±3.7 | 7.9±3.5a | 14.4±19.3 |
| 容量反应阴性组(n=21) | 9.6±3.7 | 10.3±4.0a | 9.6±15.4 | 9.6±4.2 | 8.3±3.6a | 10.8±20.5 |
| CI[L/(min·m2)] | ||||||
| 容量反应阳性组(n=24) | 2.77±0.76 | 2.44±0.69a | 11.9±7.0b | 2.76±0.70 | 3.07±0.80a | 11.6±5.2b |
| 容量反应阴性组(n=21) | 2.78±0.72 | 2.61±0.65a | 5.8±4.4 | 2.80±0.72 | 2.90±0.69a | 4.0±6.8 |
| 注:HR,心率;SBP,收缩压;MAP,平均动脉压;CVP,中心静脉压;CI,心脏指数;组内试验前后比较,aP < 0.05;与容量反应阴性组比较,bP < 0.05 | ||||||
容量反应阳性组SVV、PPV基础数值均明显高于容量反应阴性组[SVV:(10.25±2.03)% vs(8.71±2.10)%,P < 0.05;PPV:(11.58±2.93)% vs(9.52±3.11)%,P < 0.05]。
2.4 ROC曲线分析PEEP抬高试验ΔSBP、ΔCI预测容量反应性ROC曲线下面积(AUC)分别为0.737(95%CI:0.581~0.893)和0.803(95%CI:0.660~0.946)。呼气末屏气试验ΔMAP、ΔCI预测容量反应性的AUC分别为0.763(95%CI:0.617~0.908)和0.808(95%CI:0.673~0.942),均高于或与SVV(AUC=0.719, 95%CI:0.563~0.876)、PPV(AUC=0.685, 95%CI:0.526~0.843)相当。采用最大Youden指数法,PEEP抬高试验取ΔCI=12%、ΔSBP =9.5%为截断值,敏感度及特异度分别为70.8%、95.2%和75%、71.4%;呼气末屏气试验取ΔCI=8.5%、ΔMAP =5.5%为截断值,敏感度及特异度分别为79.2%、76.2%和75%、76.2%。见图 1和表 3。
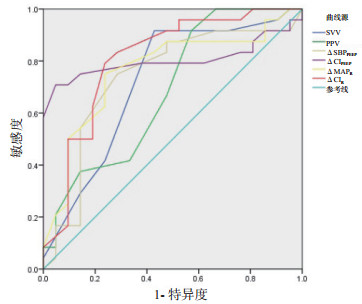
|
| ΔCIPEEP、ΔSBPPEEP为PEEP抬高试验前后CI、SBP变化;ΔCIR、ΔMAPR为呼气末屏气试验前后CI、MAP变化 图 1 各指标预测容量反应性的ROC曲线 Fig 1 ROC curve for predicting volume responsiveness by hemodynamic variables |
|
|
| 指标 | AUC (Mean±SD) |
95%CI | 截断值 (%) |
敏感度 (%) |
特异度 (%) |
| ΔCIR | 0.808±0.069 | 0.673~0.942 | 8.5 | 79.2 | 76.2 |
| ΔCIPEEP | 0.803±0.073 | 0.660~0.946 | 12.0 | 70.8 | 95.2 |
| ΔMAPR | 0.763±0.074 | 0.617~0.908 | 5.5 | 75.0 | 76.2 |
| ΔSBPPEEP | 0.737±0.080 | 0.581~0.893 | 9.5 | 75.0 | 71.4 |
| SVV | 0.719±0.080 | 0.563~0.876 | 8.5 | 91.7 | 57.1 |
| PPV | 0.685±0.081 | 0.526~0.843 | 8.5 | 91.7 | 42.9 |
| 注:ΔCIPEEP、ΔSBPPEEP为PEEP抬高试验前后CI、SBP变化;ΔCIR、ΔMAPR为呼气末屏气试验前后CI、MAP变化;AUC为受试者工作特征曲线下面积;95%CI为95%可信区间 | |||||
感染性休克血管通透性显著增加,导致有效循环血容量减少和组织灌注不足。液体复苏至关重要,但重症感染常合并心肌抑制,不当的液体复苏会导致组织水肿,影响机体氧合及组织细胞供氧,导致病死率进一步升高[12-14]。对感染性休克患者进行容量反应性监测,以指导液体复苏十分重要。通常进行容量负荷试验即500 mL液体快速静脉输注,CI增加≥15%定义为有容量反应性。液体复苏阶段常需多次判断容量反应性,而反复进行容量负荷试验存在液体负荷过多的弊端。容量反应性监测技术成为治疗感染性休克的重要研究方向。研究表明,传统的监测指标如中心静脉压、肺动脉楔压等血管内充盈压力不能作为容量反应性预测指标[15-16]。本研究中,PEEP抬高试验及呼气末屏气试验相应引起CVP升高及下降,但无论基础数值及试验前后变化,两组患者差异无统计学意义,表明CVP、ΔCVP均不能反映感染性休克患者容量反应性。
ProCESS(Protocolized Care for Early Septic Shock)[17]、ARISE(Australasian Resuscitation in Sepsis Evaluation)[18]及ProMISe(Protocolised Management in Sepsis)[19]等三项脓毒症大规模多中心试验研究结果先后发表,国际“拯救脓毒症运动”(surviving sepsis campaign,SSC)也于2017年更新指南,不再建议使用CVP、肺动脉压力等单一静态压力或容积指标反映脓毒症患者容量反应性,指南建议使用动态指标评估容量反应性,包括机械通气引起的每搏量改变等[20]。
有效循环血容量、静脉回流阻力及心肌收缩力曲线最早由Guyton[21]描述。总血容量、血流分布及外周血管阻力决定有效循环血量,驱动血液回流至心脏的上游压力为平均系统充盈压力(mean systemic pressure, Pms),右心房压力(right atrial pressure, Pra)是静脉回流的下游压力。心输出量取决于Pms与Pra之间的梯度差、静脉回流阻力,静脉回流决定并制约了心输出量。机械通气可引起胸腔内压力、肺容积变化,影响心房充盈前负荷、心室排空后负荷,影响静脉回流及心输出量[22],即为SVV、PPV、PEEP抬高试验及呼气末屏气试验(EEO)等心肺交互监测技术的生理学基础。血流动力学影响效应1 min左右即可洗脱[9-10, 23]。
机械通气引起胸腔内压力周期性改变,引起的每搏心输出量、脉搏压力变异度即SVV、PPV,作为传统的心肺交互机制监测指标,常被应用于容量反应性监测[24-25];也有研究指出各种因素可导致ICU患者SVV、PPV应用受限[26]。本研究中,容量反应阳性组SVV、PPV均明显高于容量反应阴性组[SVV:(10.25±2.03)% vs(8.71±2.10)%,P < 0.05;PPV:(11.58±2.93)% vs(9.52±3.11)%,P < 0.05],SVV、PPV预测容量反应性的AUC分别为0.719、0.685。可能是因为入选患者均为窦性心律,较深镇静能降低自主呼吸对胸腔内压力周期变化的干扰,使SVV、PPV对容量反应性的预测准确性得到提高。
Wilkman等[9]最早设计PEEP抬高试验(PEEP由10 cmH2O上调至20 cmH2O,维持60~120 s)用于感染性休克机械通气患者容量反应性监测,ROC分析ΔSBP的AUC为0.82(95%CI:0.64~1.0)。Monnet等[10]则设计了EEO试验(充分镇静实施呼气末屏气15 s),ROC分析ΔCI、ΔMAP的AUC分别为0.972(95%CI:0.849~0.995)和0.957(95%CI:0.825~0.994)。研究表明EEO也可用于肺顺应性显著下降的ARDS患者容量反应性监测[27]。Myatra等[28]发现机械通气VT 8 mL/kg比VT 6 mL/kg更有利EEO试验预测容量反应性。Georges等[29]研究EEO试验12 s结合经胸心脏超声,计算主动脉瓣时间流速积分(VTI),ROC分析示ΔVTI的AUC为0.96±0.03,ΔVTI取9%为截断值时,预测容量反应性敏感度为89%(95%CI:72%~98%),特异度为95%(95%CI:77%~100%)。本研究序贯进行PEEP抬高压力试验、EEO试验及容量负荷试验以期综合评估,研究人群均为感染性休克机械通气患者,PEEP调整方式、EEO维持时间、机械通气潮气量及镇静程度与上述研究设计略有差异。本研究表明,PEEP抬高试验ΔSBP、ΔCI预测容量反应AUC分别为0.737(95% CI:0.581~0.893)和0.803(95% CI:0.660~0.946)。呼气末屏气试验ΔMAP、ΔCI预测容量反应性的AUC分别为0.763(95% CI:0.617~0.908)和0.808(95% CI:0.673~0.942),与已有研究结果相似。PEEP抬高试验取ΔCI= 12%、ΔSBP =9.5%为截断值,敏感度及特异度分别达到70.8%和95.2%、75%和71.4%;呼气末屏气试验取ΔCI=8.5%、ΔMAP =5.5%为截断值,敏感度及特异度分别为79.2%和76.2%、75%和76.2%,均表明稍大潮气量、较深镇静均有利于提高心肺交互机制监测结果准确性。
机械通气引起肺容积改变,肺容积增加能通过血管瀑布现象引起下腔静脉膈肌入口处塌陷;而PEEP增加亦能引起上下腔静脉的塌陷,静脉回流的阻力大于Pra,且阻力位于右心房的上游,使静脉回流、CI减少,且减少不依赖Pra的变化[22]。心室收缩期室壁内外压力梯度减小,使得PEEP抬高试验时SBP降低较MAP更为明显。呼气末屏气使胸腔内压力及Pra降低,静脉回流压力梯度增加,静脉回流增加引起CI增高,舒张末期心室内外压力梯度增加,可解释MAP增高较为明显。
综上所述,PEEP抬高试验、呼气末屏气试验等基于心肺交互机制的监测技术,操作简单、安全性高、可重复进行,对感染性休克机械通气患者容量反应性具有较高预测价值。
| [1] | Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study[J]. Lancet Infect Dis, 2012, 12(12): 919-924. DOI:10.1016/S1473-3099(12)70239-6 |
| [2] | Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012[J]. JAMA, 2014, 311(13): 1308-1316. DOI:10.1001/jama.2014.2637 |
| [3] | Cuthbertson BH, Elders A, Hall S, et al. Mortality and quality of life in the five years after severe sepsis[J]. Crit Care, 2013, 17(2): R70. DOI:10.1186/cc12616 |
| [4] | Chambers KA, Park AY, Banuelos RC, et al. Outcomes of severe sepsis and septic shock patients after stratification by initial lactate value[J]. World J Emerg Med, 2018, 9(2): 113-117. DOI:10.5847/wjem.j.1920-8642.2018.02.005 |
| [5] | Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence[J]. Chest, 2002, 121(6): 2000-2008. DOI:10.1378/chest.121.6.2000 |
| [6] | 王永进, 何钢. 感染性休克液体复苏进展[J]. 中华急诊医学杂志, 2017, 26(1): 123-128. DOI:10.3760/cma.j.issn.1671-0282.2017.01.028 |
| [7] | Monnet X, Teboul JL. Assessment of volume responsiveness during mechanical ventilation: recent advances[J]. Crit Care, 2013, 17(2): 217. DOI:10.1186/cc12526 |
| [8] | 孙波, 胡雪忠, 张天卿, 等. 调节呼气末正压预测脓毒性休克患者的容量反应性研究[J]. 中华急诊医学杂志, 2016, 25(10): 1320-1323. DOI:10.3760/cma.j.issn.1671-0282.2016.10.023 |
| [9] | Wilkman E, Kuitunen A, Pettilä V, et al. Fluid responsiveness predicted by elevation of PEEP in patients with septic shock[J]. Acta Anaesthesiol Scand, 2014, 58(1): 27-35. DOI:10.1111/aas.12229 |
| [10] | Monnet X, Osman D, Ridel C, et al. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients[J]. Crit Care Med, 2009, 37(3): 951-956. DOI:10.1097/CCM.0b013e3181968fe1 |
| [11] | Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference[J]. Crit Care Med, 2003, 31(4): 1250-1256. DOI:10.1097/01.CCM.0000050454.01978.3B |
| [12] | Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality[J]. Crit Care Med, 2011, 39(2): 259-265. DOI:10.1097/CCM.0b013e3181feeb15 |
| [13] | Kelm DJ, Perrin JT, Cartin-Ceba R, et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death[J]. Shock, 2015, 43(1): 68-73. DOI:10.1097/SHK.0000000000000268 |
| [14] | 李玉婷, 李洪祥, 张东. 脓毒性休克患者容量过负荷的危险因素及预后分析[J]. 中华急诊医学杂志, 2018, 27(5): 524-528. DOI:10.3760/cma.j.issn.1671-0282.2018.05.013 |
| [15] | Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge[J]. Crit Care Med, 2007, 35(1): 64-68. DOI:10.1097/01.CCM.0000249851.94101.4F |
| [16] | Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares[J]. Chest, 2008, 134(1): 172-178. DOI:10.1378/chest.07-2331 |
| [17] | Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock[J]. N Engl J Med, 2014, 370(18): 1683-1693. DOI:10.1056/NEJMoa1401602 |
| [18] | Investigators A, Group ACT, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock[J]. N Engl J Med, 2014, 371(16): 1496-1506. DOI:10.1056/NEJMoa1404380 |
| [19] | Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock[J]. N Engl J Med, 2015, 372(14): 1301-1311. DOI:10.1056/NEJMoa1500896 |
| [20] | Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2016[J]. Crit Care Med, 2017, 45(3): 486-552. DOI:10.1097/ccm.0000000000002255 |
| [21] | Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves[J]. Physiol Rev, 1955, 35(1): 123-129. DOI:10.1152/physrev.1955.35.1.123 |
| [22] | Cherpanath TG, Lagrand WK, Schultz MJ, et al. Cardiopulmonary interactions during mechanical ventilation in critically ill patients[J]. Neth Heart J, 2013, 21(4): 166-172. DOI:10.1007/s12471-013-0383-1 |
| [23] | Biais M, Larghi M, Henriot J, et al. End-expiratory occlusion test predicts fluid responsiveness in patients with protective ventilation in the operating room[J]. Anesth Analg, 2017, 125(6): 1889-1895. DOI:10.1213/ane.0000000000002322 |
| [24] | Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation[J]. Eur J Anaesthesiol, 2012, 29(2): 64-69. DOI:10.1097/EJA.0b013e32834b7d82 |
| [25] | Latham HE, Bengtson CD, Satterwhite L, et al. Stroke volume guided resuscitation in severe sepsis and septic shock improves outcomes[J]. J Crit Care, 2017, 42: 42-46. DOI:10.1016/j.jcrc.2017.06.028 |
| [26] | Mahjoub Y, Lejeune V, Muller L, et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: a prospective observational multicentre point-prevalence study[J]. Br J Anaesth, 2014, 112(4): 681-685. DOI:10.1093/bja/aet442 |
| [27] | Monnet X, Bleibtreu A, Ferré A, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance[J]. Crit Care Med, 2012, 40(1): 152-157. DOI:10.1097/CCM.0b013e31822f08d7 |
| [28] | Myatra SN, Prabu NR, Divatia JV, et al. The changes in pulse pressure variation or stroke volume variation after a "tidal volume challenge" reliably predict fluid responsiveness during low tidal volume ventilation[J]. Crit Care Med, 2017, 45(3): 415-421. DOI:10.1097/ccm.0000000000002183 |
| [29] | Georges D, de Courson H, Lanchon R, et al. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: an echocardiographic study[J]. Crit Care, 2018, 22: 32. DOI:10.1186/s13054-017-1938-0 |
 2019, Vol. 28
2019, Vol. 28




