急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)是在严重感染、休克、创伤及烧伤等非心源性疾病过程中,因弥漫性肺间质及肺泡水肿导致的急性呼吸功能不全或衰竭。内皮细胞损伤是其主要的发病机制和病理特点[1]。活化的蛋白激酶C受体1(receptor for activated C kinase 1, RACK1)是G蛋白β亚基的同族体,可结合多种蛋白,调节细胞生理功能,参与多种疾病发生发展[2]。RACK1可与激活的蛋白激酶C(protein kinase C, PKC)、丝裂原激活蛋白激酶、c-Jun N-terminalkinase(JNK)等蛋白结合进而调控相关信号通路[3],而上述信号通路与ARDS发病密切相关[4-5]。Sonic hedgehog(SHH)信号通路主要由SHH蛋白配体、PTCH膜受体、SMOH效应器以及转录因子GLI家族组成,因其分布广泛而备受重视[6]。研究发现SHH信号通路参与ARDS发病过程[7],而RACK1参与SHH信号通路激活[8],RACK1是否参与ARDS发病及是否受SHH信号通路调控尚未见报道。本研究旨在建立脂多糖(lipopolysaccharide, LPS)致大鼠肺微血管内皮细胞(rat puhnonary microvascular endothelial cells, RPMVEC)损伤模型,探讨LPS致损过程中RACK1蛋白表达及SHH信号通路对其表达的影响。
1 材料与方法 1.1 试剂及仪器DMEM培养基(美国Hyclone公司),胎牛血清(澳大利亚Gibco公司),RACK1单克隆抗体(ab129084,英国Abcom公司)、辣根过氧化物酶标记的羊抗兔IgG(北京中杉金桥),Smoothened Agonist(SAG,SHH信号通路特异性激动剂,美国Selleck公司),免疫组化试剂盒(北京,中杉金桥),逆转录试剂盒(AT341,北京,全式金公司),梯度基因扩增仪(TProfessional PCR,德国BIOMETRA公司),防脱载玻片; 其余实验试剂均为国产分析纯试剂自行配制,SD大鼠购自安徽医科大学动物实验中心[SPF级,合格证号:SCXK(皖)2011-002]。
1.2 大鼠PMVEC分离培养及鉴定按照本实验室建立的方法及参考文献进行[9]。取SPF级SD大鼠的肺脏,体外培养RPMVEC,随机分为:(1)LPS量效组,0.1、1、10 mg/L LPS与RPMVEC孵育8 h; LPS时效组,10 mg/L LPS与RPMVEC孵育0、2、4、8、12、24 h。(2)SAG量效组,0.1、1、10 μmol/L RPMVEC孵育8 h; SAG时效组,1 μmol/L SAG与RPMVEC孵育0、2、4、8、12、24 h。(3)LPS+SAG干预组,10 mg/L LPS预孵育1 h后加入1 μmol/L SAG继续孵育8 h; 设空白组、LPS组和SAG组为对照(n=6)。
1.3 免疫细胞化学染色法检测RACK1表达将生长良好的1×105/mL RPMVEC接种于六孔板中的载玻片上,细胞生长稳定后,取出细胞爬片,4 %多聚甲醛固定,PBS冲洗,参照免疫组化试剂盒说明书操作,其中RACK1抗体稀释度为1:50,最后滴加DAB显色,自来水冲洗中止反应,苏木精轻度复染,梯度乙醇脱水,二甲苯透明后树胶封片拍照。
1.4 Western blot检测RACK1表达裂解3代RPMVEC 30 min后收集蛋白。选择10 %分离胶和5 %浓缩胶进行电泳,蛋白转移至PVDF膜上,封闭液中室温封闭2 h后,TBS-T溶液洗膜,与RACK1单克隆抗体(1:1 000)4 ℃过夜,辣根过氧化物酶标记的山羊抗兔IgG溶液(1:20 000)室温90 min孵育,自动曝光机曝光,保存条带。
1.5 逆转录聚合酶链反应法检测GLI-1 mRNA表达胰酶消化RPMVEC,Trizol一步法提取总RNA,加入无核酶水20 μL,-80 ℃保存。取溶于无核酶水中的RNA模板5 μL,70 ℃水浴变性,参照反转录试剂盒说明书完成cDNA合成。cDNA再进行PCR扩增(扩增条件为:95 ℃ 2 min预变性,95 ℃ 15 s、60 ℃ 1 min循环40次,60 ℃ 30 s,60 ℃延伸10 min)。1 %琼脂糖凝胶电泳扩增产物,凝胶成像分析系统成像。各样本的基因表达量用该样本的吸光度值与内参β-actin基因条带的吸光度值之比表示。GL I-1基因(AY695056)引物如下:GLI-1-FP:5’-CCAATCACAAGTCAGGTTCCT-3’; GLI-1-RP5’-CCTATGTGAAGCCCTATTTGCC-3’。内参β-actin-FP:5’-ATCCATCCCAATCATAGTAAC-3’,β-actin-RP:5’-CTCAAGGTCCCAACAGC-3’[10]。
1.6 实验分组和处理(1)量效实验:分别以0.1、1、10 mg/L LPS与RPMVEC孵育8 h,以0.1、1、10 μmol/L SAG与RPMVEC孵育8 h。(2)时效实验:以10 mg/L LPS与RPMVEC孵育0、2、4、8、12、24 h,以1 μmol/L SAG与RPMVEV孵育0、2、4、8、12、24 h。(3)SAG+LPS干预组:以10 mg/L LPS预孵育1 h后加入1 μmol/L SAG继续孵育8 h,设空白组、LPS组和SAG组为对照各组。干预结束后均检测RACK1蛋白及GLI-1 mRNA表达。
1.7 统计学方法采用SPSS 17.0统计学软件进行分析,计量资料以均数±标准差(x±s)表示,多组变量间比较采用单因素方差分析,组间两两比较采用SNK-q检验,以P < 0.05为差异有统计学意义。
2 结果 2.1 免疫细胞化学染色法检测RACK1在RPMVEC表达光学显微镜观察RACK1在RPMVEC细胞中表达,见图 1。
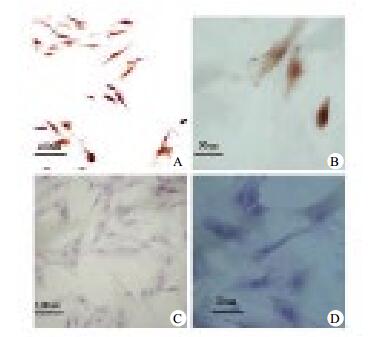
|
| 免疫细胞化学染色检测RPMVEC胞质及胞核被染成棕黄色(A:200×; B:400×); 阴性对照胞核及胞质被染成紫蓝色(C:200×; D:400×) 图 1 免疫细胞化学染色法检测RACK1在RPMVEC表达 Figure 1 Immunocytochemistry method to detect RACK1 expression in RPMVECs |
|
|
LPS未刺激时,RPMVEC低表达RACK1,0.1、1、10 mg/L LPS刺激诱导RACK1表达升高,各组间比较差异有统计学意义(P < 0.05); LPS未刺激时,RPMVEC低表达GLI-1mRNA,0.1、1、10 mg/L LPS刺激RPMVEC表达GLI-1mRNA调低,1 mg/L组与10 mg/L组比较P > 0.05,其余各组间比较差异有统计学意义(P < 0.05),见图 2、表 1。
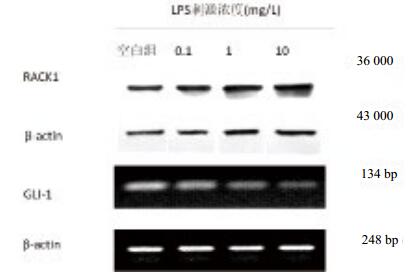
|
| LPS:脂多糖; RACK1:活化的蛋白激酶C受体1 图 2 不同浓度LPS对RPMVEC表达RACK1及GLI-1 mRNA的影响 Figure 2 Expression of RACK1 and GLI-1mRNA in RPMVECs stimulated by different concentration of LPS |
|
|
| 孵育浓度 | RACK1 | GLI-1mRNA |
| 0 mg/L | 0.354±0.025 | 1.109±0.063 |
| 0.1 mg/L | 0.451±0.025a | 1.039±0.135 |
| 1 mg/L | 0.710±0.052ab | 0.813±0.066ab |
| 10 mg/L | 1.195±0.075abc | 0.770±0.105ab |
| F值 | 312.120 | 24.321 |
| P值 | < 0.01 | < 0.01 |
| 注:与0 mg/L组比较,aP < 0.05;与0.1 mg/L组比较,bP < 0.05;与1 mg/L组比较,cP < 0.05 | ||
10 mg/L LPS刺激2 h后,RACK1表达调高,12 h达最高,24 h开始降低(图 2A),组间比较差异有统计学意义(F=1 272.204,P < 0.05); LPS刺激2 h时GLI-1 mRNA表达调低,8 h达最低,12 h后开始恢复(图 2B),组间比较差异有统计学意义(F=306.609,P < 0.05),见表 2。
| 孵育时间 | RACK1 | GLI-1mRNA |
| 0 h | 0.329±0.008 | 1.088±0.042 |
| 2 h | 0.370±0.010a | 0.929±0.007a |
| 4 h | 0.497±0.021ab | 0.890±0.017ab |
| 8 h | 0.599±0.015abc | 0.671±0.014abc |
| 12 h | 1.296±0.048abcd | 0.742±0.012abcd |
| 24 h | 0.687±0.019abcde | 0.802±0.010abcde |
| F值 | 1 272.204 | 306.609 |
| P值 | < 0.01 | < 0.01 |
| 注:与0 h组比较,aP < 0.05;与2 h组比较,bP < 0.05;与4 h组比较,cP < 0.05;与8 h组比较,dP < 0.05;与12 h组比较,eP < 0.05 | ||
0.1、1、10 μmol/L SAG刺激对各组RACK1表达无影响(均P > 0.05); SAG未刺激时RPMVEC低表达GLI-1mRNA,0 μmol/L与0.1 μmol/L比较、1 μmol/L与10 μmol/L比较GLI-1mRNA表达无统计学意义(P > 0.05),其余组间比较P < 0.05,见图 4、表 3。
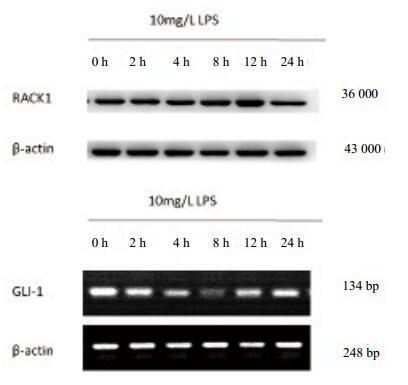
|
| LPS:脂多糖; RACK1:活化的蛋白激酶C受体1 图 3 LPS刺激不同时间诱导RPMVEC表达RACK1及GLI-1 mRNA Figure 3 Expression of RACK1 and GLI-1mRNAin RPMVEC stimulated by different lengths of time stimulated by LPS |
|
|
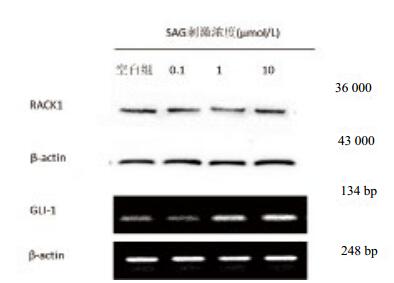
|
| LPS:脂多糖; RACK1:活化的蛋白激酶C受体1 图 4 不同浓度SAG对RPMVEC表达RACK1及GLI-1 mRNA的影响 Figure 4 Expression of RACK1 and GLI-1mRNA in RPMVEC stimulated by different concentration of SAG |
|
|
| 孵育浓度 | RACK1 | GLI-1mRNA |
| 0 μmol/L | 0.354±0.026 | 1.109±0.063 |
| 0.1 μmol/L | 0.365±0.014 | 1.169±0.052a |
| 1 μmol/L | 0.349±0.025 | 3.468±0.128ab |
| 10 μmol/L | 0.360±0.011 | 3.434±0.054ab |
| F值 | 0.733 | 1 651.006 |
| P值 | 0.544 | < 0.01 |
| 注:与0 μmol/L组比较,aP < 0.05;与0.1 μmol/L组比较,bP < 0.05 | ||
SAG对RACK1表达无影响,组间比较差异无统计学意义(F=1.140,P > 0.05)。SAG刺激2 h后GLI-1 mRNA表达调高,4 h组与8 h组比较P > 0.05,其余组间比较均P < 0.05。见表 4、图 5
| 孵育时间 | RACK1 | GLI-1mRNA |
| 0 h | 1.095±0.086 | 2.651±0.123 |
| 2 h | 1.111±0.122 | 3.027±0.065a |
| 4 h | 1.162±0.046 | 3.437±0.081ab |
| 8 h | 1.161±0.097 | 3.494±0.121ab |
| 12 h | 1.101±0.082 | 3.678±0.044abcd |
| 24 h | 1.283±0.342 | 3.796±0.086abcde |
| F值 | 1.140 | 132.841 |
| P值 | 0.360 | < 0.01 |
| 注:与0 h组比较,aP < 0.05;与2 h组比较,bP < 0.05;与4 h组比较,cP < 0.05;与8 h组比较,dP < 0.05;与12 h组比较,eP < 0.05 | ||
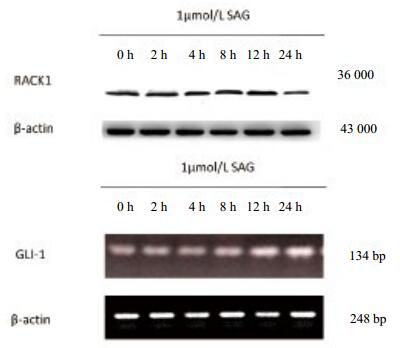
|
| LPS:脂多糖,RACK1:活化的蛋白激酶C受体1 图 5 SAG刺激不同时间诱导RPMVEC表达RACK1及GLI-1 mRNA Figure 5 Expression of RACK1 and GLI-1mRNA in RPMVEC stimulated at different lengths of time by SAG |
|
|
LPS+SAG刺激RPMVEC表达RACK1较LPS组下调,GLI-1 mRNA较LPS组上调,差异有统计学意义(P < 0.05);见表 5。SAG刺激RPMVEC表达GLI-1 mRNA与空白组比较上调,差异有统计学意义(P < 0.05),RACK1表达与空白组比较差异无统计学意义(P > 0.05),见图 6。
| 组别 | RACK1 | GLI-1mRNA |
| 空白组 | 0.703±0.020 | 2.097±0.133 |
| SAG组 | 0.729±0.055 | 3.567±0.220a |
| LPS+SAG组 | 0.831±0.040ab | 2.720±0.130ab |
| LPS组 | 1.189±0.149abc | 0.796±0.082abc |
| F值 | 44.287 | 363.274 |
| P值 | < 0.01 | < 0.01 |
| 注:与空白组比较,aP < 0.05;与SAG组比较,bP < 0.05;与LPS+SAG组比较,cP < 0.05 | ||
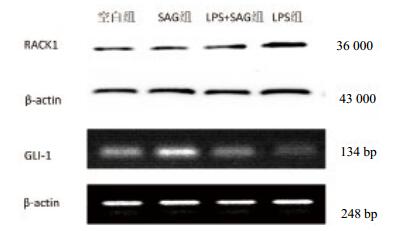
|
| LPS:脂多糖,RACK1:活化的蛋白激酶C受体1 图 6 SAG对LPS诱导RPMVEC中RACK1及GLI-1 mRNA表达的干预作用 Figure 6 Intervention effect of SAG transferase on the expression of RACK1 and GLI-1mRNA in RPMVEC induced by LPS |
|
|
RACK1为普遍表达的游离支架蛋白,有多个WD40位点,可结合PKC、cAMP-specific phosphodiesterase-4D5等蛋白[11],维持蛋白活化状态,引导其前往特定区域,介导多种信号通路激活。研究发现在大鼠脑缺血炎症致神经损伤模型中,激活星形胶质细胞的TNF-R1-FAN-RACK1-nSMase2信号通路促进中性磷脂酶积累,损伤脑神经细胞[12]; Waiskopf等[13]发现氟西汀抑制LPS诱导RACK1和PKCβⅡ过度表达,进而阻断炎症,并发现AChe-RACK1-PKCβⅡ存在交互作用; 另外,研究发现RACK1参与PKCβ激活,再作用于THP-1细胞或前白细胞,促进IL-6、IL-8和CD84生成[14]。RACK1与PKC、PDE4D5、JNK等蛋白关系密切,推测其可能通过丝裂原激活蛋白激酶、JNK、蛋白激酶A(protein kinase A, PKA)等信号通路介导LPS刺激RPMVEC。本实验发现在LPS刺激RPMVEC过程中,RACK1表达量呈时间依赖方式及浓度依赖方式增加,提示RACK1参与LPS刺激RPMVEC过程,其表达量的变化与LPS刺激变化密切相关。cAMP是细胞内重要的第二信使,可传导细胞内外各种信息,在多种炎症信号传导通路中起重要作用,cAMP可激活PKA,PKA活化亚基转位进入细胞核,促进TNF-α、IL-1-β的分泌[15]; cAMP可激活Epac蛋白,进而活化Rap1,调控细胞和细胞外基质蛋白、纤连蛋白的接触和黏连,RACK1可调控cAMP表达,故推测RACK1通过cAMP/PKA信号通路参与LPS刺激RPMVEC过程。
SHH信号通路参与如细胞增殖和保护、细胞相互作用等过程,并在炎症和组织修复过程中发挥重要作用。Yang等[7]发现LPS损伤PMVEC过程中核内GLI-1蛋白表达受到抑制,激活SHH信号通路可保护LPS致RPMVEC通透性增高。Jiang等[16]发现低密度脂蛋白损伤大鼠脑微血管内皮细胞过程中,SHH、Smo等SHH信号通路相关蛋白表达受抑,激活SHH信号通路逆转氧化低密度脂蛋白诱导的内皮细胞功能障碍,以上提示SHH通路对内皮细胞起保护性作用。本研究发现,LPS诱导GLI-1mRNA表达量成时间依赖方式及浓度依赖性下调,与Yang等[7]结果一致,间接提示SHH信号通路可保护LPS刺激RPMVEC反应。
RACK1与cAMP联系密切,通过调控cAMP表达,参与相关信号通路激活; 研究发现RACK1可结合β-actin蛋白或通过增加PDE4D5易感性调控cAMP相关信号通路[17]及PKA信号通路[11, 18]。研究证实SHH信号通路与PKA信号通路存在拮抗现象,SHH信号通路未激活时,已转录激活的GLI-1蛋白与微管蛋白结合无法进入细胞核内,会逐渐被PKA降解[19],在小脑颗粒细胞中PKA可通过抑制垂体腺苷酸环化酶多肽进而下调GLI-1mRNA表达,参与SHH信号通路调控[20],SHH信号通路可通过Ca2+通道降低纤毛内的cAMP含量进而下调PKA促进GLI-1mRNA表达[21],以上研究提示SHH信号通路和cAMP相关信号通之间可相互影响。本研究发现SHH信号通路激活可显著下调LPS诱导的RACK1高表达,但单独SHH信号通路激活并不影响RACK1表达,因此,推测SHH信号通路的保护作用可能是通过抑制LPS诱导RACK1表达量实现,因SHH信号通路与cAMP的关系推测其可能通过抑制cAMP的激活而抑制RACK1表达,但具体机制仍需要进一步探讨。
综上所述,本研究证实RACK1在RPMVEC中表达,且与LPS刺激浓度及时间关系密切,因此,推测RACK1可能参与LPS刺激RPMVEC过程; 激活SHH信号通路可下调LPS诱导的RACK1高表达,故SHH信号通路可能通过下调RACK1表达量减轻LPS刺激RPMVEC反应,因此,研究SHH信号通路与RACK1蛋白间的关系可为ARDS治疗提供新思路。
| [1] | Nelin LD, White HA, Jin Y, et al. The Src family tyrosine kinases src and yes have differential effects on inflammation-induced apoptosis in human pulmonary microvascular endothelial cells[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 310(9): L880-888. DOI:10.1152/ajplung.00306.2015 |
| [2] | Gandin V, Senft D, Topisirovic I, et al. RACK1 Function in Cell Motility and Protein Synthesis[J]. Genes Cancer, 2013, 4(9/10): 369-377. DOI:10.1177/1947601913486348 |
| [3] | Li JJ, Xie D. RACK1, a versatile hub in cancer[J]. Oncogene, 2014, 34(15): 1890-1898. DOI:10.1038/onc.2014.127 |
| [4] | Kong G, Huang X, Wang L, et al. Astilbin alleviates LPS-induced ARDS by suppressing MAPK signaling pathway and protecting pulmonary endothelial glycocalyx[J]. Int Immunopharmacol, 2016, 36: 51-58. DOI:10.1016/j.intimp.2016.03.039 |
| [5] | Lai JB, Qiu CF, Chen CX, et al. Inhibition of c-Jun N-terminal kinase signaling pathway alleviates lipopolysaccharide-induced acute respiratory distress syndrome in rats[J]. Chin Med J (Engl), 2016, 129(14): 1719-1724. DOI:10.4103/0366-6999.185867 |
| [6] | Kugler MC, Joyner AL, Loomis CA, et al. Sonic hedgehog signaling in the lung. from development to disease[J]. Am J Respir Cell Mol Bio, 2015, 52(1): 1-13. DOI:10.1165/rcmb.2014-0132TR |
| [7] | Yang Y, Li Q, Deng Z, et al. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway[J]. Mol Biol Rep, 2010, 38(6): 3615-3622. DOI:10.1007/s11033-010-0473-8 |
| [8] | Shi S, Deng YZ, Zhao JS, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway[J]. J Bio Chem, 2012, 287(11): 7845-7858. DOI:10.1074/jbc.M111.315416 |
| [9] | You QH, Sun GY, Wang N, et al. Interleukin-17F-induced pulmonary microvascular endothelial monolayer hyperpermeability via the protein kinase C pathway[J]. J Surg Res, 2010, 162(1): 110-121. DOI:10.1016/j.jss.2009.01.019 |
| [10] | 费黎明, 孙耕耘. TNF-α对大鼠肺微血管内皮细胞SSeCKS表达的影响及甲强龙的干预作用[J]. 中华肺部疾病杂志(电子版), 2012, 5(2): 105-111. DOI:10.3877/cma.j.issn.1674-6902.2012.02.003 |
| [11] | Yarwood SJ, Parnell E, Bird RJ. The cyclic AMP phosphodiesterase 4D5 (PDE4D5)/receptor for activated C-kinase 1 (RACK1) signalling complex as a sensor of the extracellular nano-environment[J]. Cell Signal, 2017, 35: 282-289. DOI:10.1016/j.cellsig.2017.01.013 |
| [12] | Gu L, Huang B, Shen W, et al. Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi[J]. J Neuroinflammation, 2013, 10: 109. DOI:10.1186/1742-2094-10-109 |
| [13] | Waiskopf N, Ofek K, Gilboa-Geffen A, et al. AChE and RACK1 promote the anti-inflammatory properties of fluoxetine[J]. J Mol Neurosci, 2013, 53(3): 306-315. DOI:10.1007/s12031-013-0174-6 |
| [14] | Corsini E, Galbiati V, Papale A, et al. The role of HSP27 in RACK1-mediated PKC activation in THP-1 cells[J]. Immunol Res, 2016, 64(4): 940-950. DOI:10.1007/s12026-016-8802-1 |
| [15] | 尤青海, 孙耕云, 高磊, 等. src抑制的蛋白激酶C底物调控内皮细胞分泌TNF-α[J]. 中华急诊医学杂志, 2012, 21(12): 1349-1353. DOI:10.3760/cma.j.issn.1671-0282.2012.12.012 |
| [16] | Jiang XL, Chen T, Zhang X. Activation of sonic hedgehog signaling attenuates oxidized low-density lipoprotein-stimulated brain microvascular endothelial cells dysfunction in vitro[J]. Int J Clin Exp Pathol, 2015, 8(10): 12820-12828. |
| [17] | Hetman M, Neasta J, Fiorenza A, et al. Activation of the cAMP pathway induces rack1-dependent binding of β-actin to bdnf promoter[J]. Plos One, 2016, 11(8): e0160948. DOI:10.1371/journal.pone.0160948 |
| [18] | Bolger GB. RACK1 and beta-arrestin2 attenuate dimerization of PDE4 cAMP phosphodiesterase PDE4D5[J]. Cell Signal, 2016, 28(7): 706-712. DOI:10.1016/j.cellsig.2015.08.003 |
| [19] | Liem KF, He M, Ocbina PJR, et al. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling[J]. Proc Natl Acad Sci U S A, 2009, 106(32): 13377-13382. DOI:10.1073/pnas.0906944106 |
| [20] | Niewiadomski P, Zhujiang A, Youssef M, et al. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA[J]. Cell Signal, 2013, 25(11): 2222-2230. DOI:10.1016/j.cellsig.2013.07.012 |
| [21] | Moore BS, Stepanchick AN, Tewson PH, et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics[J]. Proc Natl Acad Sci U S A, 2016, 113(46): 13069-13074. DOI:10.1073/pnas.1602393113 |
 2018, Vol. 27
2018, Vol. 27




