奥普力农是1996年在日本上市的PDE Ⅲ抑制剂。首先在麻醉犬中被证实可增加心脏的收缩力,减少了外周血管的阻力,但不影响平均动脉压和心率[1-3]。奥普力农药理作用较为广泛,可抑制心肌细胞与血管平滑肌PDE Ⅲ的活性,兼有正性肌力和血管扩张作用[4-5],相比米力农具有优势[6],目前在国内主要应用于心力衰竭治疗[7]。
缺血-再灌注损伤的发生机制尚未完全阐明,一般认为,线粒体通透性转换孔的开放(mPTP),细胞内pH变化,细胞内钙超载和由活性氧(ROS)产生的氧化应激以及由细胞因子和补体系统介导的炎症[8-9]。研究[10-14]发现心肌损伤时自噬可以保护心肌,但也有研究发现自噬可损害心肌[15-16],故自噬减轻还是加重心肌损伤还有争议[17]。
奥普力农对心肌缺血-再灌注的预处理可使心输出量显著提高和再灌注后的左心室舒张末压和全身血管阻力指数显著降低,同时,也能可有效的降低肌酸激酶和肌钙蛋白[18],减少心肌缺血梗死面积[19],具有抗炎作用等[20]。现有研究均是预处理给药,而在临床工作中常常由于不能预测缺血的发生时间而限制了预处理的应用,而更贴近临床实践的奥普力农后处理对心功能、凋亡、自噬的影响鲜见报道。本研究拟探讨奥普力农后处理能否适当抗凋亡、调节自噬,从而保护心肌。
1 材料与方法 1.1 实验动物及试剂10周大的雄性SD大鼠,体质量约280~350 g,购自上海实验动物中心。奥普力农由河北爱尔海泰制药有限公司生产提供。抗Caspase-3抗体、抗Bax抗体、抗Lc3抗体购自美国Abcam公司,抗Bcl-2抗体购自Immunoway公司,抗Beclin-1抗体、抗GAPDH抗体购自Santa Cruz公司,HRP标记的二抗购自美国Bioworld公司。
1.2 心肌缺血-再灌注模型雄性SD大鼠分别用三种剂量(0.2 mg, 0.6 mg,2 mg/kg i.p.q12 h)的奥普力农缺血-再灌注后处理,用戊巴比妥钠(45 mg/kg i.p.)麻醉大鼠。于胸骨左旁3、4肋间开胸暴露心脏,剪开心包,结扎冠状动脉左前降支中1/3,以结扎的心肌组织颜色变灰作为结扎成功的标志,结扎30 min后释放缝线再灌注,同时注射第一次奥普力农,12 h后再注射第二次奥普力农,共再灌注24 h[21]。将存活的大鼠分为5组:(1)sham组。大鼠开胸,心肌穿过缝线,但未结扎,(n=6);(2)I/R组。大鼠结扎,但未注射奥普力农,(n=9);(3)奥普力农低剂量组。大鼠结扎,腹腔注射0.2 mg/kg q12 h,(n=6);(4)奥普力农中剂量组。大鼠结扎,腹腔注射0.6 mg/kg q12 h,(n=6);(5)奥普力农高剂量组。大鼠结扎,腹腔注射2 mg/kg q12 h,(n=6)。
1.3 心肌功能检测心肌缺血-再灌注24 h后,用心脏功能分析系统(上海奥尔科特生物科技有限公司)检测心脏血流动力学指标。分离出大鼠左侧股动脉和右侧颈动脉,分别置入硬脊膜外麻醉导管。检测动脉收缩压、最大左室压力变化速度、左室舒张末压等反应心肌功能的血流动力学指标。
1.4 心肌梗死百分比计算心功能检测之后,取出心脏,用1%的伊文氏蓝4 mL左右进行冠脉灌注,沿结扎点处取心脏组织横切面(1~2 mm厚),放入装有PBS的培养皿中,加入适量TTC,室温下放置10 min后取出。心脏横切面中蓝色区域被伊文氏蓝染色,代表未受损心肌,红色区域代表心肌缺血区,缺血区中被TTC染成白色区域为心肌梗死区,心脏梗死的百分比即为梗死区与缺血区的比值。
1.5 各组间24 h内病死率对比24 h内病死率指第一次注射奥普力农后成功关胸至24 h后测心功能之前的死亡个数除以这期间死亡个数+成功存活满24 h个数。
1.6 Western印记分析收集研碎的心肌组织,细胞裂解液裂解心肌获得蛋白匀浆,BCA法定量蛋白,蛋白质印迹法检测Caspase-3、Bax、Bcl-2、LC3B、LC3A、Beclin-1等蛋白的表达。用Image Pro Plus6.0图像分析软件处理,以目的条带与内参GAPDH条带的灰度值比表示蛋白的相对含量。
1.7 统计学方法采用SPSS 22.0统计软件,计量资料经正态性检验及方差齐性检验,符合正态分布采用均数±标准差(x±s)进行统计描述,不同组之间的符合正态分布及方差齐性的计量数据采用ANOVA,两两比较采用LSD-t方法,计数资料以百分比表示,多组率的比较采用R×C表的卡方检验,以P < 0.05为差异有统计学意义。
2 结果 2.1 奥普力农改善缺血-再灌注损伤心肌的功能SD大鼠再灌注24 h后,用心脏功能分析系统检测心脏血流动力学指标。如表 1所示,与sham组相比,I/R组和I/R+Olprinone-L组的Pmax显著下降, 差异有统计学意义(t=2.91, P < 0.01;t=2.98, P < 0.01);与I/R组相比,I/R+Olprinone-M组使Pmax升高(t=2.30, P < 0.05)。与sham组相比,I/R组和I/R+Olprinone-L组的Pmin增加, 差异有统计学意义(t=3.35, P < 0.01;t=2.90, P < 0.01)。与I/R组相比,I/R+Olprinone-M组使Pmin显著下降(t=3.18, P < 0.01)。与sham组相比,I/R组的LVEDP显著增加, 差异有统计学意义(t=2.47, P < 0.05), I/R+Olprinone-M组的LVEDP显著下降(t=2.36, P < 0.05);与I/R组相比,I/R+Olprinone-M组与I/R+Olprinone-H组使LVEDP显著下降(t=5.06, P < 0.01:t=4.34, P < 0.01)。dP/dtmax:与sham组比较,I/R组的dP/dtmax明显下降(t=5.24, P < 0.01);与I/R组相比,I/R+Olprinone-M组、I/R+Olprinone--H组的dP/dtmax均有不同程度升高(t=4.18, P < 0.01:t=1.78, P < 0.05)。-dP/dtmin:与sham组比较,I/R组-dP/dtmin下降明显(t=4.95, P < 0.01);与I/R组相比,I/R+Olprinone-M组的-dP/dtmin明显升高,差异有统计学意义(t=3.34, P < 0.01)。SAP、MAP、DAP、Pmean差异无统计学意义(P > 0.05)。
| 指标 | 假手术组 | 缺血-再灌注组 | 奥普力农低剂量组 | 奥普力农中剂量组 | 奥普力农高剂量组 |
| SAP(mmHg) | 91.79±2.81 | 83.99±4.683 | 80.43±5.17 | 85.91±7.58 | 87.39±6.418 |
| DAP(mmHg) | 79.14±3.34 | 63.48±7.81 | 51.03±3.82 | 53.66±7.60 | 58.71±6.87 |
| MAP(mmHg) | 84.99±2.68 | 76.50±6.111 | 64.00±3.85 | 70.01±8.54 | 73.16±7.28 |
| Pmax(mmHg) | 100.13±6.12 | 77.78±6.41a | 75.07±4.96a | 95.42±3.83b | 87.49±4.73 |
| Pmin(mmHg) | 10.65±2.28 | 30.30±5.09a | 29.27±1.97a | 11.67±3.33b | 24.32±5.75 |
| Pmean(mmHg) | 56.25±2.65 | 50.33±5.80 | 53.17±2.85 | 49.44.±4.72 | 54.37±5.75 |
| LVEDP(mmHg) | 32.79±2.49 | 45.06±3.83a | 43.56±1.88 | 20.00±4.41ab | 23.51±4.26b |
| dp/dtmax(mmHg/s) | 3 333.73±513.59 | 1 348.29±88.67a | 1 268.98±146.24a | 2 934.51±372.00b | 2 022.43±201.43ab |
| dp/dtmin(mmHg/s) | 3 198.93±578.08 | 1 163.23±196.06a | 1 229.32±188.52a | 2 537.95±279.28b | 1 784.50±171.03a |
| 注:SAP (收缩压),DAP (舒张压) Pmax (左室最大发展压),Pmin (左室最小发展压),Pmean (平均左室发展压),LVEDP (左室舒张末压),+dp/dtmax (左室压力上升的最大变化率),-dp/dtmin (左室压力下降的最大变化率);与sham组比较,aP < 0.05;与I/R组比较,bP < 0.05 | |||||
如图 1所示,与I/R组比较(63.66±5.89)%,I/R+Olprinone-M组、I/R+Olprinone-H组的心肌梗死面积百分比分别为(42.66±2.94)%、(22.33.44±3.63)%,差异有统计学意义(t=3.13, P < 0.01:t=6.16, P < 0.01), I/R+Olprinone-L组(65.50±6.83)%,差异无统计学意义(t=0.27, P > 0.05)。
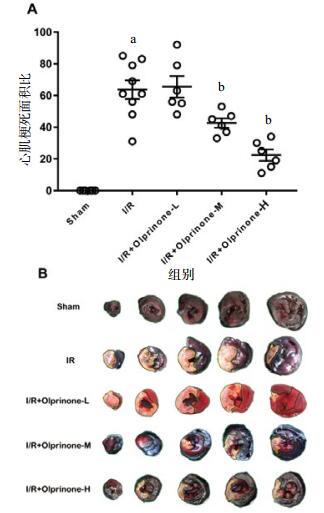
|
| A心肌梗死面积百分比:即梗死区与缺血区的比值。B大鼠左心室缺血-再灌注心肌横切面;绿色虚线表示未缺血或梗死区域;黑色虚线表示缺血+梗死区域;黄色虚线表示梗死区域。数据以x±s表示。与sham组比较,aP < 0.01;与I/R组比较,bP < 0.05 图 1 各组大鼠心肌梗死面积百分比(%) Figure 1 Percentage of myocardial infarction area in each group |
|
|
如图 2所示,实验各组之间24 h内的病死率对比,差异无统计学意义(P > 0.05)。
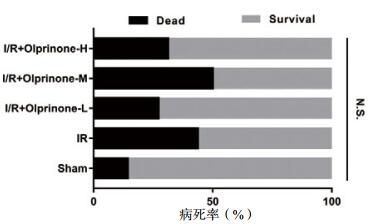
|
| 图 2 各组大鼠24 h内的病死率 Figure 2 Rats mortality in each group within 24 hours |
|
|
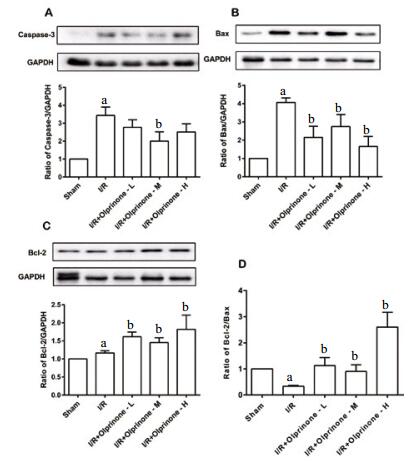
|
| A:Caspase-3、GAPDH的显影图像和Caspase-3与内参蛋白GAPDH的比值;B:Bax、GAPDH的显影图像;Bax与内参蛋白GAPDH的比值;C:Bcl-2、GAPDH的显影图像;Bcl-2与内参蛋白GAPDH的比值;D:Bcl-2与Bax的比值。数据以x±s表示,与sham组比较,aP < 0.05;与I/R组比较,bP < 0.05 图 3 不同剂量的奥普力农对各组凋亡蛋白表达的影响 Figure 3 Effect of different doses of olprinone on the expression of apoptosis related proteins in each group |
|
|
用westerblot免疫印迹法分析奥普力农对凋亡蛋白表达的影响,结果显示与sham组相比,I/R组显著增加了Caspase-3表达,差异有统计学意义(3.44±0.47,t=5.58, P < 0.01);olprinone-M组Caspase-3表达为(2.00±0.52,t=2.06,P < 0.05), 低于I/R组;olprinone-L组、olprinone-H组表达为(2.77±0.42,t=1.06,P > 0.05;2.51±0.47,t=1.40,P > 0.05), 差异无统计学意义。与sham组相比,I/R组能使Bax表达增加(4.06±0.25,t=14.35, P < 0.01);olprinone-L组、olprinone-M组、olprinone-H组下调Bax表达为(2.16±0.61,t=2.903, P < 0.01;2.74±0.66,t=2.01, P < 0.05;1.65±0.55,t=4.26, P < 0.01)。与sham组相比, I/R组上调Bcl-2表达(1.17±0.06,t=2.92, P < 0.01)olprinone-L组、olprinone-M组、olprinone-H组能不同程度的促进Bcl-2表达(1.62±0.13,t=3.15, P < 0.01;1.46±0.13,t=1.99, P < 0.05;1.82±0.39,t=1.97, P < 0.05)。由Bcl-2与Bax蛋白表达计算得出Bcl-2/Bax比值,与sham组相比,I/R组Bcl-2/Bax比值降低(0.33±0.03, t=21.5,P < 0.01);olprinone-L组、olprinone-M组、olprinone-H组预处理使其不同程度升高,差异有统计学意义(1.13±0.31,t=2.78, P < 0.01;1.46±0.25,t=2.24, P < 0.05;2.60±0.57,t=4.80,P < 0.01)。
2.5 对大鼠缺血-再灌注中Beclin-1蛋白、Bcl-2/Beclin-1比值,LC3B/LC3A比值的影响与sham组Beclin-1蛋白水平比较,I/R组Beclin-1蛋白表达量升高(1.43±0.06, t=2.46, P < 0.05);olprinone-L组、olprinone-M组Beclin-1的表达均高于I/R组(2.46±0.44,t=2.50, P < 0.05;2.80±0.75,t=2.194, P < 0.05), 差异有统计学意义,olprinone-H组表达量为(2.23±0.68,t=1.263,P > 0.05),差异无统计学意义。由Bcl-2与Beclin-1蛋白表达计算得出Bcl-2/ Beclin-1比值,与sham组相比,I/R组Bcl-2/Beclin-1比值未有降低(1.22±0.44,t=1.37,P > 0.05);olprinone-L组、olprinone-M组、olprinone-H组预处理使其不同程度降低,但只有olprinone-M组差异有统计学意义(0.89±0.69,t=1.46,P > 0.05;0.63±0.13,t=2.50,P < 0.05;0.87±0.20,t=1.86,P > 0.05)。相比Sham组,I/R组升高LC3B/LC3A比值(1.36±0.31,t=1.59,P > 0.05),olprinone-L组、olprinone-M组、olprinone-H组LC3B/LC3A比值分别为(3.84±0.52,t=3.92,P < 0.01;3.22±0.67,t=2.50,P < 0.05;2.53±0.58,t=2.36,P < 0.05),差异有统计学意义,但olprinone-M组LC3B/LC3A比值居中。
3 讨论心肌缺血-再灌注使心肌细胞超微结构、代谢和电生理损伤[22-24],引起血管和心肌的炎症[25];激活心肌细胞凋亡和自噬机制亡[20-21]。本研究发现奥普力农可明显改善缺血-再灌注损伤的心肌功能,通过抗凋亡,调节自噬平衡减少心肌损伤。
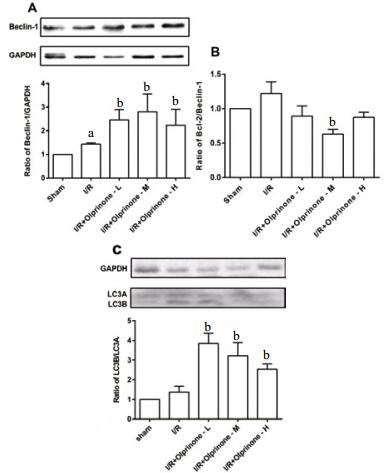
|
| A:Beclin-1、GAPDH的显影图像和Beclin-1与内参蛋白GAPDH的比值;B:Bcl-2与Beclin-1的比值;C:LC3B、LC3A显影图像和LC3B、LC3A的比值;数据以x±s表示,与sham组比较,aP < 0.05;与I/R组比较,bP < 0.05 图 4 不同剂量的奥普力农对各组自噬相关蛋白表达及Bcl-2/Beclin-1比值的影响 Figure 4 Effects of different doses of olprinone on the expression of autophagy related proteins and the ratio of Bcl-2/Beclin-1 in each group |
|
|
心肌缺血-再灌注损伤容易导致心力衰竭,造成血流动力学紊乱,甚至影响全身脏器灌注,正性肌力药物能否有效的增强心肌细胞功能、不增加心肌细胞氧耗、改善血流动力学显得异常重要。我们的心功能数据显示奥普力农可以增加心脏Pmax(左室最大发展压)、+dp/dtmax(左室压力最大上升变化率)、-dp/dtmin(左室压力最大下降变化率),降低Pmin (左室最小发展压)、LVEDP (左室舒张末压)等心脏缩舒功能指标,发现在中剂量组效果最佳,可能奥普力农正性肌力及舒张心肌作用与其在心脏冠脉中的浓度有关[26]。奥普力农对血压影响不明显,提示奥普力农可以增强缺血-再灌注损伤的心肌功能,不会导致血流动力学紊乱。
由于心肌细胞是不可再生的细胞,心肌细胞凋亡在心脏处于病理状态时出现,如过负荷或心肌缺血[27]。研究认为BcL-2/Bax比值对凋亡有决定作用[28-29],奥普力农预处理可降低Bax、增强Bcl-2表达,从而抑制大鼠缺血-再灌注心肌细胞凋亡[20]。此外,细胞凋亡可通过外源性或内源性通路汇聚到caspase-3[30];在大鼠心脏移植缺血-再灌注模型中也证实,阻断caspase-3能减轻再灌注损伤[31]。结果发现奥普力农后处理能抑制促凋亡蛋白Bax和Caspase-3表达,增强抗凋亡蛋白Bcl-2表达,提高Bcl-2与Bax的比值,表明奥普力农通过调节促凋亡和抗凋亡信号通路平衡,达到保护心肌功能的作用。
自噬是一种重要的代谢过程,在机体出现癌症,缺血,感染等病理性压力时被激活,包括在溶酶体中对不必要或功能失调的细胞成分进行降解和再循环利用[32-33]。有学者证实大鼠和小鼠心脏急性缺血-再灌注后可见自噬的激活[34-35], 研究也表明心肌缺血再灌损伤时能激活自噬[21]。再灌注阶段过度自噬也会损害心肌[16],抑制自噬可保护心肌[15, 36],故可能调节自噬适当水平能发挥最大的心肌保护作用。此外,自噬与凋亡之间存在紧密联系,自噬对细胞存亡的决定往往取决于凋亡蛋白Bcl-2和自噬蛋白Beclin-1之间的平衡,Bcl-2/Beclin-1是判断自噬调节的重要标准,Bcl-2下调能通过Beclin-1维持自噬适当水平,防止自噬致细胞死亡[37]。研究表明Bcl-2/Beclin-1比值下调可增强大鼠脑梗死后的神经保护[38],但Bcl-2/Beclin-1在心肌缺血-再灌注中的研究鲜有报道。发现中剂量奥普力农使Bcl-2与Beclin-1的比值最小,Bcl-2能通过与Beclin-1的相互作用调节自噬,抑制自噬过度诱导细胞死亡,最大限度地发挥保护心肌作用。
| [1] | Ogawa T, Ohhara H, Tsunoda H, et al. Cardiovascular effects of the new cardiotonic agent 1, 2-dihydro-6-methyl-2-oxo-5-(imidazo[1, 2-a]pyridin-6-yl)-3-pyridine carbonitrile hydrochloride monohydrate. 1st communication: studies on isolated guinea pig cardiac muscles[J]. Arzneimittelforschung, 1989, 39(1): 33-37. |
| [2] | Ohhara H, Ogawa T, Takeda M, Katoh H, Daiku Y, Igarashi T. Cardiovascular effects of the new cardiotonic agent 1, 2-dihydro-6-methyl-2-oxo-5-(imidazo[1, 2-a]pyridin-6-yl)-3-pyridine carbonitrile hydrochloride monohydrate. 2nd communication: studies in dogs[J]. Arzneimittelforschung, 1989, 39(1): 38-45. |
| [3] | Satoh H, Endoh M. Effects of a new cardiotonic agent 1, 2-dihydro-6-methyl-2-oxo-5-[imidazo (1, 2-a) pyridin-6-yl]-3-pyridine carbonitrile hydrochloride monohydrate (E-1020) on contractile force and cyclic AMP metabolism in canine ventricular muscle[J]. Jpn J Pharmacol, 1990, 52(2): 215-224. DOI:10.1254/jjp.52.215 |
| [4] | Takaoka H, Takeuchi M, Odake M, et al. Comparison of the effects on arterial-ventricular coupling between phosphodiesterase inhibitor and dobutamine in the diseases human heart[J]. J Am Coll Cardiol, 1993, 22(2): 598-606. |
| [5] | Mizushige K, Ueda T, Yuk Ⅱ ri K, et al. Olprinone: a phosphodiesterase Ⅲ inhibitor with positive inotropic and vasodilator effects[J]. Cardiovasc Drug Rev, 2002, 20(3): 163-174. DOI:10.1111/j.1527-3466.2002.tb00085.x |
| [6] | Dobashi S, Watanabe I, Matsumoto S, et al. Comparative e ffect of milrinone and oplprinone in patients with congestive heart failure[J]. J Am Col Card, 2016, 67(13): 1420-1420. DOI:10.1016/S0735-1097(16)31421-8 |
| [7] | 奥普力农在急性心力衰竭中应用共识专家组. 奥普力农在急性心力衰竭中应用的专家共识[J]. 中华急诊医学杂志, 2016, 25(5): 573-576. DOI:10.3760/cma.j.issn.1671-0282.2016.05.007 |
| [8] | Arumugam TV, Okun E, Tang SC, et al. Toll-like receptors in ischemia-reperfusion injury[J]. Shock, 2009, 32(1): 4-16. DOI:10.1097/SHK.0b013e318193e333 |
| [9] | Fröhlich GM, Meier P, White SK, et al. Myocardial reperfusion injury: looking beyond primary PCI[J]. Eur Heart J, 2013, 34(23): 1714-1722. DOI:10.1093/eurheartj/eht090 |
| [10] | Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium[J]. Heart Fail Rev, 2007, 12(34): 307-317. |
| [11] | Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. Ⅰ. Ultrastructural and cytochemical changes[J]. Am J Pathol, 1980, 98(2): 425-444. |
| [12] | Khan S, Salloum F, Das A, et al. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes[J]. J Mol Cell Cardiol, 2006, 41(2): 256-64. DOI:10.1016/j.yjmcc.2006.04.014 |
| [13] | Huang C, Yitzhaki S, Perry CN, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection[J]. J Cardiovasc Transl Res, 2010, 3(4): 365-373. DOI:10.1007/s12265-010-9189-3 |
| [14] | Gurusamy N, Lekli I, Gorbunov NV, et al. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein[J]. J Cell Mol Med, 2009, 13(2): 373-387. DOI:10.1111/j.1582-4934.2008.00495.x |
| [15] | Valentim L, Laurence KM, Townsend PA, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury[J]. J Mol Cell Cardiol, 2006, 40(6): 846-852. |
| [16] | Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy[J]. Circ Res, 2007, 100(6): 914-922. |
| [17] | Li T, Jiao YR, Wang LH, et al. Autophagy in myocardial ischemia reperfusion injury: Friend or foe?[J]. Int J Cardiol, 2017, 239: 10. DOI:10.1016/j.ijcard.2017.01.083 |
| [18] | Setoyama K, Kamimura R, Fujiki M, et al. Effects of olprinone on myocardial ischemia-reperfusion injury in dogs[J]. J Vet Med Sci, 2006, 68(8): 865-868. DOI:10.1292/jvms.68.865 |
| [19] | Tosaka S, Makita T, Tosaka R, et al. Cardioprotection induced by olprinone, a phosphodiesterase Ⅲ inhibitor, involves phosphatidylinositol-3-OH kinase-Akt and a mitochondrial permeability transition pore during early reperfusion[J]. J Anesth, 2007, 21(2): 176-801. DOI:10.1007/s00540-006-0485-7 |
| [20] | Di Paola R, Mazzon E, Paterniti I, et al. Olprinone, a PDE3 inhibitor, modulates the inflammation associated with myocardial ischemia-reperfusion injury in rats[J]. Eur J Pharm, 2011, 650(23): 612-20. DOI:10.1016/j.ejphar.2010.10.043 |
| [21] | Zhang GX, Kimura S, Murao K, et al. Inhibition of cytochrome c release by 10-N-nonyl acridine orange, a cardiolipin-specific dye, during myocardial ischemia-reperfusion in the rat[J]. Am J Physiol Heart Circ Physiol, 2010, 298(2): H433-439. DOI:10.1152/ajpheart.00938.2009 |
| [22] | Cabrera-Fuentes HA, Aragones J, Bernhagen J, et al. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: meeting report from the third international symposium on "New frontiers in cardiovascular research"[J]. Basic Res Cardiol, 2016, 111(6): 69. DOI:10.1007/s00395-016-0586-x |
| [23] | Donnarumma E, Ali MJ, Rushing AM, et al. Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability[J]. J Am Heart Assoc, 2016, 5(7): pii: e003531. DOI:10.1161/JAHA.116.003531 |
| [24] | Yang Y, Ma Z, Hu W, et al. Caveolin-1/-3: therapeutic targets for myocardial ischemia/reperfusion injury[J]. Basic Res Cardiol, 2016, 111(4): 45. DOI:10.1007/s00395-016-0561-6 |
| [25] | Yellon DM, Hausenloy DJ. Myocardial reperfusion injury[J]. N Engl J Med, 2007, 357(11): 1121-1135. DOI:10.1056/NEJMra071667 |
| [26] | Fuj Ⅱ Y, Toyooka H. Different effects of olprinone on contractility in nonfatigued and fatigued diaphragm in dogs[J]. Can J Anaesth, 2000, 47(12): 1243-1248. DOI:10.1007/BF03019875 |
| [27] | McCully JD, Wakiyama H, Hsieh Y-J, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury[J]. Am J Physiol Heart Circ Physiol, 2004, 286(5): H1923-1935. DOI:10.1152/ajpheart.00935.2003 |
| [28] | Love S. Apoptosis and brain ischaemia[J]. Prog Neuropsychopharmacol Biol Psychiatry, 2003, 27(2): 267-282. DOI:10.1016/S0278-5846(03)00022-8 |
| [29] | Yan P, Chen SQ, Li ZP, et al. Effect of exogenous phosphocreatine on cardiomycytic apoptosis and expression of Bcl-2 and Bax after cardiopulmonary resuscitation in rats[J]. World J Emerg Med, 2011, 2(4): 291-295. DOI:10.5847/wjem.j.1920-8642.2011.04.009 |
| [30] | Almeida A. Genetic determinants of neuronal vulnerability to apoptosis[J]. Cell Mol Life Sci, 2013, 70(1): 71-88. DOI:10.1007/s00018-012-1029-y |
| [31] | Grünenfelder J, Miniati DN, Murata S, et al. Upregulation of Bcl-2 through caspase-3 inhibition ameliorates ischemia/reperfusion injury in rat cardiac allografts[J]. Circulation, 2001, 104(12 Suppl 1): I202-I206. DOI:10.1161/hc37t1.094833 |
| [32] | Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense[J]. Cell, 2005, 120(2): 159-162. DOI:10.1016/j.cell.2005.01.005 |
| [33] | Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease[J]. Annu Rev Pathol, 2013, 8: 105-137. DOI:10.1146/annurev-pathol-020712-163918 |
| [34] | Hamacher-Brady A, Brady NR, Gottlieb RA, et al. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart[J]. Autophagy, 2006, 2(4): 307-309. DOI:10.4161/auto.2947 |
| [35] | Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy[J]. Cell Death Differ, 2007, 14(1): 146-57. DOI:10.1038/sj.cdd.4401936 |
 2018, Vol. 27
2018, Vol. 27




