快速、准确地评估批量伤员的病情,及时高效地进行救治,是急诊科所要解决的重要问题之一。尤其是面对突发公共事件时,短时间内及时准确的分检出危重伤员并开展救治,对降低致残率和致死率都至关重要。针对批量伤员的检伤分类,目前损伤严重度评分(injury severity score,ISS评分)[1]应用较为广泛。但该评分方法采用解剖部位为基础进行评分计算,不能反映伤员的生理变化,存在一定的局限性。D-二聚体是纤溶酶作用于交联蛋白的特异性分子标志物,是凝血功能相关疾病常用的检测指标,在创伤患者中也常有升高[2-3]。本文通过回顾性研究1 167例创伤患者的临床资料,探讨D-二聚体联合ISS评分预测创伤患者预后的意义。
1 资料与方法 1.1 一般资料采用回顾性分析方法,选择南京军区南京总医院急诊抢救室在2014年1月1日至2016年12月31日收治的创伤患者共计1 592例。排除标准:(1)年龄 < 14岁、伤后时间> 24 h、入院时已死亡、自动离院及数据不完整者;(2)既往有凝血功能障碍性疾病者;(3)既往有原发性肝功能损害者;(4)受伤前6个月内曾使用抗凝药物者;(5)受伤部位 < 2处。最终纳入1 167例创伤患者。所有检查检验及抢救治疗措施均获得患者及家属的知情同意,按照创伤治疗的指南进行治疗。
1.2 方法对所有纳入患者的临床资料进行回顾性分析,统计患者入院时ISS评分[4]及首次静脉血血浆D-二聚体值,D-二聚体使用日本希森美康(Sysmex)CA-7000全自动凝血分析仪检测,测定采取免疫比浊法。根据ISS评分结果,将患者分为轻伤组(ISS ≤ 16分),中度创伤组(16分 < ISS ≤ 25分)和重度创伤组(ISS > 25分)。以28 d预后为观察终点将患者分为存活组及死亡组。比较不同分组D-二聚体与患者病死率之间的关系。通过ROC曲线下面积,评估ISS评分以及D-二聚体联合ISS评分对患者预后预测的意义。
1.3 统计学方法采用SPSS 17.0统计学软件进行分析,定量资料以均数±标准差(x ± s)、中位数(四分位数)表示,非正态分布的样本组间采用两样本Mann-Whitney U检验或多样本Kruskal-Wallis单因素ANOVA检验表示,正态分布的样本组间比较采用完全随机设计独立样本t检验或单因素方差分析;定性资料采用例数和率(%)表示,组间比较采用χ2检验;采用趋势性χ2检验比较不同评分分组中D-二聚体、病死率的变化趋势;采用Pearson相关分析探究D-二聚体值与ISS评分的相关性。根据患者的ISS评分及血D-二聚体值,应用MedCalc12.1统计软件生成受试者工作特征(ROC)曲线,采用DeLong-DeLong非参数法比较各项评分ROC曲线下总面积,分析ISS评分、D-二聚体及两者联合后对创伤患者预后的预测价值。ROC曲线下总面积在0.5~0.7时预测价值较低,>0.7且≤0.9时预测价值中等,>0.9时预测价值较高[5]。以P < 0.05为差异有统计学意义。
2 结果 2.1 纳入患者的一般资料最终纳入1 167例患者,男性865例,女性302例;年龄(48.45±17.21)岁。将所有患者按预后分为存活组和死亡组,存活组1 092例(93.6%),死亡组75例(6.4%),见表 1。
| 指标 | 存活组(n=1 092) | 死亡组(n=75) | t/χ2值 | P值 |
| 性别(男/女) | 811/281 | 54/21 | 0.188 | 0.665 |
| 年龄(岁) | 47.72±16.99 | 59.09±16.94 | -5.571 | 0.000 |
| 致伤因素(例,%) | 11.023a | 0.012a | ||
| 车祸伤 | 550(50.4) | 47(62.7) | ||
| 高处坠落 | 162(14.8) | 13(17.3) | ||
| 摔伤 | 129(11.8) | 10(13.3) | ||
| 打架斗殴 | 87(8.0) | 1(1.3) | ||
| 重物砸伤 | 30(2.7) | 0(0) | ||
| 爆炸或烧伤 | 20(1.8) | 0(0) | ||
| 电击伤 | 9(0.8) | 0(0) | ||
| 其他 | 105(9.6) | 4(5.3) | ||
| 注:a表示卡方检验时将致伤因素为打架斗殴、重物砸伤、爆炸或烧伤、电击伤和其他合并为一组进行计算 | ||||
存活组患者的ISS评分和D-二聚体水平明显低于死亡组,差异有统计学意义(P < 0.01),见表 2。
| 组别 | 例数 | ISS评分 [分,M(IQR)] |
D-二聚体 [mg/L, M(IQR)] |
| 存活组 | 1 092 | 5(2, 9) | 7.14(1.24, 18.00) |
| 死亡组 | 75 | 9(6, 25) | 34.26(9.95, 40) |
| Z值 | -6.694 | -7.777 | |
| P值 | < 0.01 | < 0.01 |
不同评分分组间病死率及D-二聚体差异有统计学意义(P < 0.01),同时趋势性检验显示随着ISS评分增加,D-二聚体值及病死率均有上升趋势(Ptrend < 0.01),见表 3。
| ISS评分(分) | 例数 | 存活(n=1092) | 死亡(n=75) | 病死率(%) | D-二聚体(mg/L, M(IQR)) |
| ≤16 | 1 068(91.5) | 1 018(93.2) | 50(66.7) | 4.91 | 7.23(1.23, 17.97) |
| 16~25 | 83(7.1) | 64(5.9) | 19(25.3) | 29.69 | 18.38(7.05, 40.00) |
| >25 | 16(1.4) | 10(0.9) | 6(8) | 60 | 24.58(5.40, 40.00) |
| χ2值 | 70.85 | 43.26 | |||
| P值 | < 0.01 | < 0.01 | |||
| Ptrend值 | < 0.01 | 0.01 |
Pearson相关分析显示,急诊创伤患者D-二聚体与ISS评分呈正相关(r = 0.342,P < 0.01),见图 1。
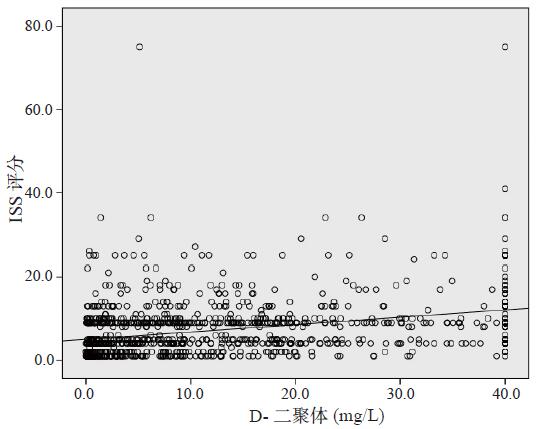
|
| 图 1 D-二聚体与ISS评分值的相关散点图 Figure 1 Scatter plot of D-dimer versus ISS |
|
|
ISS评分、D-二聚体以及ISS评分联合D-二聚体对患者病死率预测性能进行比较,生成ROC曲线,并对曲线下面积进行比较(图 2)。
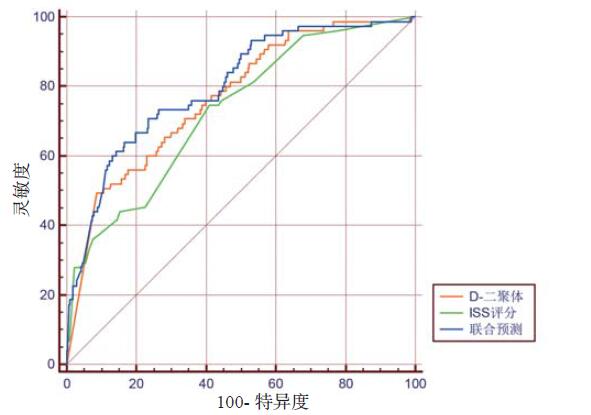
|
| 图 2 ISS评分、D-二聚体及两者联合预测患者预后受试者工作特征(ROC)曲线 Figure 2 ROC curve of ISS, D-dimer and joint both to predict prognosis in trauma patients |
|
|
ISS评分、D-二聚体以及ISS评分联合D-二聚体对患者预后都有一定的预测能力,ISS评分联合D-二聚体后对患者预后的判断能力最优。D-二聚体单独预测患者预后的ROC曲线下面积大于ISS评分,但之间的差异无统计学意义(Z=1.051,P=0.293);两者联合预测患者预后的ROC曲线下面积大于ISS评分、D-二聚体单独预测面积(Z=3.028,Z=2.272,P < 0.05),见表 4。
| 预测指标 | AUC | 误差 | 95%CI |
| ISS评分 | 0.728 | 0.029 6 | 0.702~0.754 |
| D-二聚体 | 0.765 | 0.028 2 | 0.739~0.789 |
| 两者联合预测 | 0.800 | 0.026 8 | 0.776~0.822 |
批量伤员的检伤分类是急诊救治的第一步,快速、准确、有效地评估伤员的轻重程度,对指导急诊医师采取有效的救治措施,减少伤员创伤后遗症、降低致残率和致死率,提高急诊救治效率和质量有重要意义。目前国内使用较多的是损伤严重度评分(ISS评分)。ISS评分是在简明损伤分级(abbreviated injury scale, AIS)评分基础上修正和改进而来,通过对创伤患者不同部位伤情的判定,计算出最终的分值,分值高低不仅代表了创伤严重程度,而且对创伤患者死亡风险的评估有重要价值。近期国内一项包含93例严重多发伤患者的研究显示,ISS评分预测患者病死率的ROC曲线下面积为0.897[6],显示了较好的预测效能。国内外均有较多研究证实,ISS评分是患者死亡的独立影响因素[7-9],其分值越高,创伤患者死亡风险越大[10],患者的病死率随其升高而升高。来自泰国的研究者在一项前瞻性队列研究中表明,患者病死率与ISS评分呈正相关,高ISS评分组中有更多患者需直接采取手术治疗,且高龄、低血压、以及格拉斯哥评分等影响患者病死率的相关参数也与高ISS评分相关联[7]。
但ISS评分也存在较大局限性。因为ISS评分对创伤患者进行评定时以解剖部位为基础,对患者的年龄、病理生理情况、以及全身的严重反应等因素的影响并未考虑进去,对患者伤后的生理功能紊乱状况并未反映出来,由于每个患者存在个体差异,对创伤的承受能力及治疗的反应性不尽相同,临床表现也呈现多样性,患者解剖部位的损伤严重程度与其创伤后病理生理改变状况并不一致。同时ISS评分对相同的受伤区域的多处损伤只取一处,不能真实反映多脏器伤的严重性;对腹腔内的复杂脏器相互区别不够;不能很准确的反映颅脑损伤的严重程度[11]。另外,患者入院后,需要经过一系列检查检验明确诊断,对一些脏器、血管、神经的损伤则需要进行手术后才能明确诊断,影响了ISS评分的准确度。
D-二聚体是纤溶酶降解交联蛋白的特异性产物,反映了凝血系统激活后的纤维蛋白溶解状态[12],既往有研究表明,纤维蛋白溶解,是创伤诱导的凝血病和凝血功能损伤的重要组成部分,并且可导致患者预后不良[13-17]。D-二聚体升高可见于严重创伤、感染、肿瘤以及脑梗死、肺栓塞等疾病,近期国内研究显示D-二聚体对主动脉夹层的初筛性诊断以及预后的预测也有重要应用价值[18]。严重创伤引起的休克和组织低灌注,可造成患者血管内膜受损,内膜下组织暴露,进而激活凝血系统,使血液患者处于高凝状态,同时诱导内皮细胞组织型纤溶酶原激活物(t-PA)的急性释放[19]引起纤溶亢进。严重创伤患者在高动力循环、高代谢作用下,机体内的纤溶和凝血系统发生紊乱,而机体释放的大量炎性介质,也可以激活凝血系统,使体内微血栓广泛的形成,都使D-二聚体异常持续升高,较重的患者最终会导致多器官障碍功能综合征、甚或多器官功能衰竭而死亡[20-21]。
严重创伤后出现呼吸窘迫综合征、多器官功能衰竭、弥散性血管内凝血等并发症,诊断时可以使用D-二聚体作为参考因素[22],而且国内外均有较多研究表明,D-二聚体与创伤患者的预后显著相关。Tian等[23]及Tong等[24]的研究报道了较高D-二聚体水平的脑创伤患者与其不良预后相关。亦有研究表明,所有严重创伤患者的高D-二聚体水平与预后不良以及组织损伤的严重程度相关[25-29]而不论有无合并脑损伤[17, 30-31]。
D-二聚体检测操作简便,较短时间即可得到结果,价格也较为低廉,评估患者凝血功能时常被作为首选化验指标。近年来,血栓弹力图在临床应用亦较为广泛,常被用来描记患者的凝血功能状态。有研究显示,血栓弹力图检测出纤溶亢进的创伤患者中,可以观察到D-二聚体水平是增高的,但在D-二聚体增高的创伤患者中血栓弹力图检测并不都会出现纤溶亢进的结果[30]。因此,本研究选用更为方便快捷且廉价的D-二聚体作为预测指标,与ISS评分联合判断创伤患者的预后。本研究依据ISS评分将所有创伤患者分为不同创伤程度组,结果评分越高的分组患者病死率越高,且血D-二聚体水平也越高,D-二聚体与ISS评分呈现正相关,与其他国内外学者研究结果相符[32-33]。将ISS评分、D-二聚体以及两者联合对患者预后预测性能进行比较,结果显示ISS评分、D-二聚体以及两者联合判断的ROC曲线下面积均大于0.5,均有诊断意义,且两者联合后诊断强度显著大于单用ISS评分及D-二聚体判断患者预后[34]。
综上所述,ISS评分与血D-二聚体均与创伤患者的病情严重程度相关,ISS评分增高、血D-二聚体升高都提示创伤患者死亡风险增高,两者均能预测患者预后,ISS评分和血D-二聚体存在相关性,且两者联合后预测性能大于单独预测。由于各种评分在预测创伤患者预后方面存在局限性,本研究通过评分及血液学检查指标联合对创伤患者预后进行预测,探讨评估创伤患者病情危重程度及预后的新思路。对急诊医师接诊创伤患者并及时采取干预,降低患者病死率,具有重要临床指导意义。
| [1] | Baker SP, O'Neill B, Haddon W, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care[J]. J Trauma, 1974, 14(3): 187-196. DOI:10.1097/00005373-197403000-00001 |
| [2] | Magetsari R, Dewo P, Nugroho AS, et al. Deep vein thrombosis in elderly patients following surgery for fracture of the proximal femur[J]. Malays Orthop J, 2014, 8(3): 7-10. DOI:10.5704/MOJ.1411.002 |
| [3] | Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia[J]. Anesth Analg, 2009, 108(3): 751-758. DOI:10.1213/ane.0b013e3181966675 |
| [4] | Paffrath T, Lefering R, Flohé S, et al. How to define severely injured patients? -- an Injury Severity Score (ISS) based approach alone is not sufficient[J]. Injury, 2014, 45(Suppl 3): S64-69. DOI:10.1016/j.injury.2014.08.020 |
| [5] | 宋花玲, 贺佳, 黄品贤, 等. ROC曲线下面积估计的参数法与非参数法的应用研究[J]. 第二军医大学学报, 2006, 27(7): 726-728. DOI:10.3321/j.issn.0258-879X.2006.07.008 |
| [6] | 陈小凤, 阳文新, 孙守松, 等. ISS评分与CRAMS评分在多发伤患者预后评估中的应用[J]. 中华急诊医学杂志, 2017, 26(6): 664-668. DOI:10.3760/cma.j.issn.1671-0282.2017.06.013 |
| [7] | Wuthisuthimethawee P. Trauma team activation criteria in managing trauma patients at an emergency room in Thailand[J]. Eur J Trauma Emerg Surg, 2017, 43(1): 53-57. DOI:10.1007/s00068-015-0624-7 |
| [8] | Sarani B, Temple-Lykens B, Kim P, et al. Factors associated with mortality and brain injury after falls from the standing position[J]. J Trauma, 2009, 67(5): 954-958. DOI:10.1097/TA.0b013e3181ae6d39 |
| [9] | 陈卫强, 李辉, 马岳峰, 等. 浙江省8家医院创伤患者死亡危险因素分析[J]. 中华急诊医学杂志, 2011, 20(3): 297-301. DOI:10.3760/cma.j.issn.1671-0282.2011.03.021 |
| [10] | 李兵, 阮海林, 葛文汉, 等. 创伤指数在评估急性创伤住院患者病情及预后的应用研究[J]. 中国急救医学, 2014, 34(1): 71-74. DOI:10.3969/j.issn.1002-1949.2014.01.017 |
| [11] | 高劲谋. 多发伤和创伤评分[J]. 中华创伤杂志, 2007, 23(3): 161-163. DOI:10.3760/j.issn.1001-8050.2007.03.001 |
| [12] | Wada H, Sakuragawa N. Are fibrin-related markers useful for the diagnosis of thrombosis?[J]. Semin Thromb Hemost, 2008, 34(1): 33-38. DOI:10.1055/s-2008-1066021 |
| [13] | Theusinger OM, Wanner GA, Emmert MY, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma[J]. Anesth Analg, 2011, 113(5): 1003-1012. DOI:10.1213/ANE.0b013e31822e183f |
| [14] | Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma[J]. Ann Surg, 2010, 252(3): 434-442. DOI:10.1097/SLA.0b013e3181f09191 |
| [15] | Schochl H, Frietsch T, Pavelka M, et al. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry[J]. J Trauma, 2009, 67(1): 125-131. DOI:10.1097/TA.0b013e31818b2483 |
| [16] | Theusinger OM, Baulig W, Seifert B, et al. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department[J]. Anesth Analg, 2015, 120(3): 627-635. DOI:10.1213/ANE.0000000000000561 |
| [17] | Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients[J]. J Thromb Haemost, 2013, 11(2): 307-314. DOI:10.1111/jth.12078 |
| [18] | 薛渊, 肖子亚, 顾国嵘, 等. D-二聚体对急性主动脉夹层诊断及预后判断价值[J]. 中华急诊医学杂志, 2017, 26(8): 935-938. DOI:10.3760/cma.j.issn.1671-0282.2017.08.021 |
| [19] | Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system[J]. J Thromb Haemost, 2009, 7(1): 4-13. DOI:10.1111/j.1538-7836.2008.03220.x |
| [20] | Berger RP, Fromkin J, Rubin P, et al. Serum D-dimer concentrations are increased after pediatric traumatic brain injury[J]. J Pediatr, 2015, 166(2): 383-388. DOI:10.1016/j.jpeds.2014.10.036 |
| [21] | 邓银灿, 周华, 符一骐, 等. 降钙素原、C反应蛋白及D-二聚体在预测重症肺炎患者预后中的作用[J]. 上海交通大学学报(医学版), 2014, 34(9): 1372-1375, 1385. DOI:10.3969/j.issn.1674-8115.2014.09.022 |
| [22] | Schreiber MA, Differding J, Thorborg P, et al. Hypercoagulability is most prevalent early after injury and in female patients[J]. J Trauma, 2005, 58(3): 475-480. DOI:10.1097/01.TA.0000153938.77777.26 |
| [23] | Tian HL, Chen H, Wu BS, et al. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases[J]. Neurosurg Rev, 2010, 33(3): 359-365. DOI:10.1007/s10143-010-0251-z |
| [24] | Tong WS, Zheng P, Zeng JS, et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury[J]. Brain Inj, 2012, 26(9): 1136-1142. DOI:10.3109/02699052.2012.666437 |
| [25] | Oshiro A, Yanagida Y, Gando S, et al. Hemostasis during the early stages of trauma: comparison with disseminated intravascular coagulation[J]. Crit Care, 2014, 18(2): R61. DOI:10.1186/cc13816 |
| [26] | Hayakawa M, Sawamura A, Gando S, et al. Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase[J]. Surgery, 2011, 149(2): 221-230. DOI:10.1016/j.surg.2010.06.010 |
| [27] | Sawamura A, Hayakawa M, Gando S, et al. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality[J]. Thromb Res, 2009, 124(5): 608-613. DOI:10.1016/j.thromres.2009.06.034 |
| [28] | Hayakawa M, Maekawa K, Kushimoto S, et al. High d-dimer levels predict a poor outcome in patients with severe trauma, even with high fibrinogen levels on arrival: a multicenter retrospective study[J]. Shock, 2016, 45(3): 308-314. DOI:10.1097/SHK.0000000000000542 |
| [29] | Yuan F, Ding J, Chen H, et al. Predicting outcomes after traumatic brain injury: the development and validation of prognostic models based on admission characteristics[J]. J Trauma Acute Care Surg, 2012, 73(1): 137-145. DOI:10.1097/TA.0b013e31824b00ac |
| [30] | Kutcher ME, Cripps MW, McCreery RC, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma[J]. J Trauma Acute Care Surg, 2012, 73(1): 87-93. DOI:10.1097/TA.0b013e3182598c70 |
| [31] | Ostrowski SR, Sørensen AM, Larsen CF, et al. Thrombelastography and biomarker profiles in acute coagulopathy of trauma: a prospective study[J]. Scand J Trauma Resusc Emerg Med, 2011, 19: 64. DOI:10.1186/1757-7241-19-64 |
| [32] | Zhu YJ, Huang XK. Relationship between disseminated intravascular coagulation and levels of plasma thrombinogen segment 1+2, D-dimer, and thrombomodulin in patients with multiple injuries[J]. Chin J Traumatol, 2009, 12(4): 203-209. |
| [33] | Rangarajan K, Subramanian A, Gandhi JS, et al. Coagulation studies in patients with orthopedic trauma[J]. J Emerg Trauma Shock, 2010, 3(1): 4-8. DOI:10.4103/0974-2700.58652 |
| [34] | Hagiwara S, Oshima K, Aoki M, et al. Usefulness of fibrin degradation products and d-dimer levels as biomarkers that reflect the severity of trauma[J]. J Trauma Acute Care Surg, 2013, 74(5): 1275-1278. DOI:10.1097/TA.0b013e31828cc967 |
 2018, Vol. 27
2018, Vol. 27




