2. 天津医科大学基础医学院药理学系,天津 300070;
3. 天津医科大学第二医院检验科,天津 300211
2. Department of Pharmacology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China;
3. Department of Clinical Laboratory, The Second Hospital of Tianjin Medical University, Tianjin 300211, China
急性肺损伤(acute lung injury,ALI)是一种危及生命的疾病,其特征是失控的炎症反应和血管通透性增加[1],其机制包括肺上皮和内皮的损伤、氧化应激的损伤、促炎介质的产生和大量中性粒细胞募集入肺等 [2-4]。而氧化应激增强炎症细胞的募集和激活,从而导致炎症反应级联放大,从而导致细胞损伤[5]。研究表明,ABL激酶在炎症和血管渗漏中发挥作用。伊马替尼是第一个获得FDA批准的ABL激酶抑制剂,已被证明在许多氧化剂诱导的损伤模型中具有保护作用 [7],但具体机制尚未确定。核因子红细胞2相关因子2/血红素氧合酶-1 (Nrf2/HO-1)信号通路是抗氧化应激的重要信号通路,损伤激活后可调节抗氧化物质的释放,从而抑制氧化应激[8-9]。本研究通过建立内毒素血症急性肺损伤小鼠模型,探讨伊马替尼能否通过核因子红细胞2相关因子2/血红素氧合酶-1 (Nrf2/HO-1)信号通路减轻氧化应激减轻内毒素性急性肺损伤。
1 材料与方法本研究经天津医科大学动物伦理与福利委员会批准,审查编号TMUaMEC 2023002。伊马替尼购自美国Abmole公司,大肠杆菌O111:B4产LPS购自美国Sigma-Aldrich公司,TNF-α及IL-6 Elisa试剂盒购自美国Invitrogen,Nrf2、HO-1购自美国CST公司,p-NF-κB、β-actin、山羊抗兔二抗购自美国abcam公司,丙二醛(malondialdehyde,MDA) 检测试剂盒、谷胱甘肽(glutathione, GSH)检测试剂盒、过氧化氢酶(catalase, CAT)检测试剂盒、超氧化物歧化酶(superoxide dismutase, SOD)检测试剂盒和还原型谷胱甘肽/氧化型谷胱甘肽(GSH/GSSG)检测试剂盒均购自南京建成生物,RIPA裂解液购自武汉Servicebio公司。
选用60只体重20~25 g的SPF级6~8周龄雄性C57BL/6小鼠。将小鼠用随机数字表法分为4组(每组n=15): 对照组(C组)、伊马替尼组(I组)、内毒素血症组(LPS组)、伊马替尼+内毒素血症组(I+LPS组)。
采用腹腔注射LPS 15 mg/kg制备内毒素血症急性肺损伤小鼠模型,C组与I组腹腔注射等量生理盐水。伊马替尼溶解于10%的DMSO中,I+LPS组于造模前30 min、I组于相应时点,尾静脉注射伊马替尼40 mg/kg,C组与LPS组同一时点尾静脉注射等量生理盐水。造模24 h后,进行样本取材。
取新鲜右肺组织,称重后80 ℃烘箱连续烘干48 h后再次称重,计算前后两次即肺湿重∕干重(W∕D)比值。取左肺组织,4%多聚甲醛固定48 h,进行石蜡包埋和切片,HE染色后,光镜下观察肺组织病理改变并进行病理评分。
1.1 ELISA检测TNF-α及IL-6水平取小鼠心尖血,离心机4℃预冷,3 500 r/min离心15 min后取上清液,根据ELISA试剂盒说明书,测定血清TNF-α和IL-6水平,在酶标仪波长450 nm上读取吸光度值。
1.2 检测肺组织中MDA、SOD、T-GSH/GSSH、CAT、LDH水平取肺组织,称重后按1∶9的比例加入磷酸盐缓冲液(phosphate buffered sawater,PBS),剪碎离心,制备成10%匀浆上清液; 按试剂盒说明书步骤测定MDA、SOD、GSH、CAT、LDH吸光度值,然后计算其含量。
1.3 Western blot检测取右肺组织,采用Western blot法检测核转录因子红系2相关因子2 (Nrf2)及血红素氧合酶1(HO-1)蛋白表达水平。肺组织称重,剪碎,加入RIPA裂解液、蛋白酶抑制剂和磷酸酶抑制剂,4℃下12 000 r/min离心15 min,离心半径5 cm,收集上清液,BCA法测定蛋白浓度。按1:4比例加入4x蛋白上样缓冲液,SDS-聚丙烯酰胺凝胶电泳分离目的蛋白,冰浴转膜后,应用含0.1% Tween-20和5%脱脂牛奶的TBS缓冲液室温封闭印迹膜1 h,洗膜后加入一抗Nrf2抗体(1:1000)、HO-1抗体(1:1000)、p-NF-κB(1:1000)4℃孵育过夜。TBST洗膜3次,加入过氧化物酶标记的二抗(1:5000),室温孵育2 h,TBST洗膜3次后加入荧光化学发光液显影,用Image J软件分析图像,目的蛋白相对表达量以目的蛋白条带灰度值与β-actin条带灰度值的比值表示。
1.4 统计学方法采用SPSS20.0软件进行分析,正态分布的计量资料以均数±标准差(x±s)表示,组间比较采用单因素方差分析,计数资料比较采用χ2检验,以P > 0.05为差异有统计学意义。
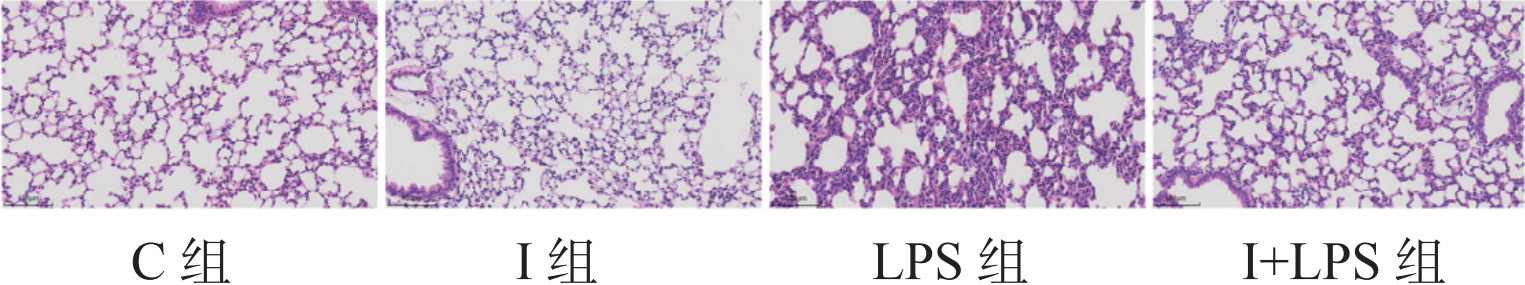
2 结果 2.1 四组小鼠病理损伤情况与C组和I组相比,LPS组小鼠肺部色泽暗红,分布大小不一的出血点,偶见散在斑块状出血,切面可见泡沫状淡红色液体流出:I+LPS组较LPS组损伤情况略轻。光镜下:C组和I组肺组织未见明显异常;LPS组可见肺泡结构严重破坏,肺间质增宽,肺泡腔有出血、水肿,大量中性粒细胞浸润;I+LPS组小鼠肺间质增宽,肺泡腔出血、水肿,中性粒细胞浸润较LPS组减轻;C组与I组比较,差异无统计学意义(P > 0.05)。见图 1。与C组相比,LPS组及I+LPS组肺组织W/D比值及肺损伤评分升高(P > 0.05),I组上述指标差异无统计学意义(P > 0.05)。与LPS组比较,I+LPS肺组织W/D比值及肺损伤评分降低(P > 0.05),见表 1。
|
| 图 1 光镜下四组小鼠肺组织病理学结果(HE染色×200) Fig 1 The pathology results of mice lung tissue under light microscopy in the four groups(HE staining x200) |
|
|
| 组别 | 肺W/D比值 | 肺损伤评分(分) |
| C组 | 3.47±0.41 | 1.25±0.89 |
| I组 | 3.75±0.06 | 1.50±0.76 |
| LPS组 | 5.58±0.47 | 10.25±1.75 |
| I+LPS组 | 4.62±0.38 | 7.00±1.31 |
| F值 | 20.00 | 100.50 |
| P值 | < 0.0001 | < 0.0001 |
| 注:W/D比值为湿重/干重比值 | ||
与C组相比,LPS组和I+LPS组小鼠血清中的TNF-α和IL-6水平明显升高(P > 0.05),I组差异无统计学意义(P > 0.05)。与LPS组比较,I+LPS组TNF-α和IL-6水平显著降低(P > 0.05),见表 2。
| 组别 | IL-6 | TNF-α |
| C组 | 12.31±3.14 | 34.07±5.01 |
| I组 | 15.77±2.93 | 48.76±0.91 |
| LPS组 | 1909.00±88.73 | 124.50±37.20 |
| I+LPS组 | 778.50±33.10 | 65.23±4.33 |
| F值 | 91.64 | 22.04 |
| P值 | P < 0.0001 | P < 0.0001 |
| 注:IL-6为白细胞介素; TNF-α为肿瘤坏死因子α | ||
与C组相比,LPS组丙二醛(MDA)表达显著升高,过氧化氢酶(CAT)、超氧化物歧化酶(SOD)和谷胱甘肽(GSH)表达显著降低(P < 0.05),I+LPS组给予药物治疗后均有显著恢复(P < 0.05),见表 3。
| 组别 | MDA(μmol/g) | SOD (U/mgprot) | CAT (U/mgprot) | GSH(μmol/g) | GSH/GSSG |
| C组 | 1.05±0.11 | 23.01±3.38 | 22.73±1.08 | 13.07±0.65 | 3.83±0.32 |
| I组 | 1.14±0.13 | 17.36±2.56 | 19.81±0.77 | 10.40±0.80 | 3.63±0.23 |
| LPS组 | 5.55±0.70 | 5.07±1.14 | 12.92±1.26 | 4.03±0.52 | 2.31±0.21 |
| I+LPS组 | 3.08±0.80 | 14.10±1.79 | 16.31±0.85 | 6.83±1.09 | 3.05±0.07 |
| F值 | 76.84 | 50.10 | 88.83 | 124.6 | 46.55 |
| P值 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| 注:MDA为丙二醛,SOD为超氧化物歧化酶,CAT为过氧化氢酶,GSH为还原型谷胱甘肽,GSH/GSSG为还原型谷胱甘肽/氧化型谷胱甘肽 | |||||
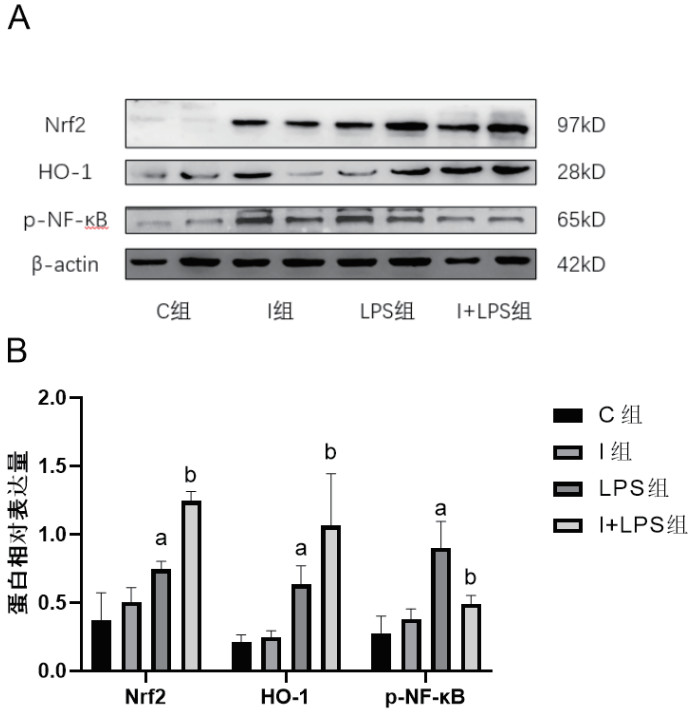
与C组相比,I组Nrf2、HO-1和p-NF-κB表达差异无统计学意义(P > 0.05),LPS组和I+LPS组Nrf2和HO-1表达上调,p-NF-κB升高(P > 0.05)。与LPS组相比,I+LPS组Nrf2和HO-1表达水平更为升高,p-NF-κB水平下降(P > 0.05)。见图 2。
|
| 与C组比较,aP < 0.05;与LPS组比较,bP < 0.05 图 2 四组小鼠肺组织Nrf2、HO-1、p-NF-κB蛋白表达及相对表达水平 Fig 2 Expression and the relative expression of Nrf2、HO-1、p-NF-κB in mice lung tissue in the four groups |
|
|
急性肺损伤特征是肺血管通透性增加、肺水肿、中性粒细胞过度迁移以及促炎细胞因子和介质释放[10]。急性肺损伤的常见原因是严重感染,主要由革兰氏阴性菌细胞壁中的LPS引起[11]。本研究参照文献[12]的方法,采用腹腔注射LPS 15 mg/kg制备内毒素血症急性肺损伤模型,并于造模后24 h检测。本研究显示,造模后24 h,小鼠出现呼吸频率加快、活动度下降、眼部分泌物增多、寒战、毛发树立等表现,病理切片显示肺泡正常结构消失,肺间隔增厚,肺血管壁通透性严重破坏,炎症细胞浸润,同时血清IL-6、TNF-α等炎症因子及肺组织氧化应激指标升高,提示内毒素血症急性肺损伤模型建立成功。
本研究发现,在LPS诱导的脓毒症急性肺损伤小鼠中,炎症因子释放增加,氧化应激产物MDA水平升高,抗氧化酶SOD、GSH水平降低,NF-κB、Nrf2和HO-1表达上调。伊马替尼处理后,炎症因子产生减少,氧化应激损伤减轻。伊马替尼是第一个获得美国食品药品监督管理局(FDA)批准的酪氨酸激酶抑制剂,最初是通过抑制BCR-Abl融合蛋白来治疗慢性粒细胞白血病的,它还作用于c-Abl、abl相关基因(Arg)等多种激酶。既往研究表明,它对人类和实验动物也有有益的药理作用,如抗炎、抗纤维化和改善血管渗漏作用[13-16]。Abl位点活化可以激活NF-κB通路,在LPS激活炎症细胞和随后的TNF-α产生中起重要作用[17];在内毒素血症小鼠模型中,抑制Abl激酶可调节中性粒细胞外陷阱形成[18],减少炎症细胞因子水平[19],NF-κB激活介导促炎细胞因子如TNF-α、IL-1β和IL-6的转录,进而增强炎症反应,从而促进ALI和其他炎性疾病的发展[20]。笔者前期研究证明[21],Abl和Src双靶点抑制剂博舒替尼可以通过减轻炎症损伤,来减轻内毒素急性肺损伤。NF-κB可调节各种免疫反应和不同炎性细胞因子的表达。NF-κB可调节IL-6和TNF-α的表达。本研究中,伊马替尼预处理显著抑制小鼠IL-6、TNF-α的产生。
Nrf2是调控氧化应激的重要转录因子,氧化应激是许多疾病的共同特征,包括神经退行性疾病、心血管疾病、气道疾病和一些病毒感染。Nrf2在多种组织中表达,小鼠的肠道、肺和肾脏中表达最丰富。Nrf2其特异性受体Kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein 1, Keap1)在氧化应激损伤时参与活化[22]。在正常状态下,Keap1与Nrf2相互作用,抑制Nrf2的活性。机体受到刺激发生氧化应激损伤,活性氧会修饰Keap1蛋白的半胱氨酸残基,使其释放Nrf2,导致Nrf2的积累及其向细胞核的易位,与抗氧化反应元件(anti - oxide response elements, ARE)结合,启动下游抗氧化酶的转录和表达,包括HO-1和SOD和GSH,进而保护机体免受氧化应激的损伤[22-25]。在本研究中,LPS显著诱导氧化应激,Nrf2和HO-1的表达升高。此外已有证据表明Nrf2/HO-1的激活可以调节其对NF-κB的抑制同时NF-κB在转录水平上可以抑制了Nrf2信号传导。抑制NF-κB,可增加HO-1 mRNA和蛋白表达[25]。而LPS刺激会诱导Nrf2和NF-κB的激活[26],两种转录因子不太可能完全相互作用。在本研究中,伊马替尼治疗后p-NF-κB表达下降,Nrf2和HO-1表达升高。总之,伊马替尼可改善炎症,减轻氧化应激状态,Nrf2和HO-1表达上调,氧化应激产物MDA水平降低,抗氧化酶SOD、GSH水平增高,炎症因子释放减少,肺组织损伤减轻。
综上所述,伊马替尼可改善内毒素血症急性肺损伤,其作用机制可能是通过改善炎症和抗氧化应激来发挥保护作用。本研究也存在不足之处,Nrf2与NF-κB的相互作用,伊马替尼在Nrf2的上游机制尚不明确,后续可通过敲低或过表达方式对其抗氧化通路进行进一步验证。
利益冲突 所有作者声明无利益冲突
作者贡献声明 李端阳、刘雅茹、杨红:课题实施、论文撰写;周芷晴、宗晓龙:数据整理、统计学分析;李真玉:研究设计、实验指导、论文修改、经费支持
| [1] | Artham S, Gao F, Verma A, et al. Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury[J]. Pharmacol Res, 2019, 141: 249-263. DOI:10.1016/j.phrs.2019.01.006 |
| [2] | Spinelli E, Mauri T, Beitler JR, et al. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions[J]. Intensive Care Med, 2020, 46(4): 606-618. DOI:10.1007/s00134-020-05942-6 |
| [3] | Tasaka S, Amaya F, Hashimoto S, et al. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome[J]. Antioxid Redox Signal, 2008, 10(4): 739-753. DOI:10.1089/ars.2007.1940 |
| [4] | Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome[J]. N Engl J Med, 2017, 377(6): 562-572. DOI:10.1056/nejmra1608077 |
| [5] | Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease[J]. Kidney Int Suppl, 2008(111): S4-S9. DOI:10.1038/ki.2008.516 |
| [6] | Wang J, Pendergast AM. The emerging role of ABL kinases in solid tumors[J]. Trends Cancer, 2015, 1(2): 110-123. DOI:10.1016/j.trecan.2015.07.004 |
| [7] | Akashi N, Matsumoto I, Tanaka Y, et al. Comparative suppressive effects of tyrosine kinase inhibitors imatinib and nilotinib in models of autoimmune arthritis[J]. Mod Rheumatol, 2011, 21(3): 267-275. DOI:10.1007/s10165-010-0392-5 |
| [8] | Ali T, Kim T, Rehman SU, et al. Natural dietary supplementation of anthocyanins via PI3K/akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease[J]. Mol Neurobiol, 2018, 55(7): 6076-6093. DOI:10.1007/s12035-017-0798-6 |
| [9] | Wei CC, Kong YY, Li GQ, et al. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway[J]. Sci Rep, 2017, 7(1): 717. DOI:10.1038/s41598-017-00851-z |
| [10] | Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment[J]. J Aerosol Med Pulm Drug Deliv, 2010, 23(4): 243-252. DOI:10.1089/jamp.2009.0775 |
| [11] | Tsai CL, Lin YC, Wang HM, et al. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats[J]. J Ethnopharmacol, 2014, 153(1): 197-206. DOI:10.1016/j.jep.2014.02.010 |
| [12] | Kim KH, Kwun MJ, Choi JY, et al. Therapeutic effect of the Tuber of Alisma orientale on lipopolysaccharide-induced acute lung injury[J]. Evid Based Complement Alternat Med, 2013, 2013: 863892. DOI:10.1155/2013/863892 |
| [13] | Li P, Liang QL, Cui XD, et al. Protective effects of the active fraction from the tuber of Scirpus yagara in mouse endotoxin shock model[J]. J Ethnopharmacol, 2014, 158 Pt A: 331-337. DOI:10.1016/j.jep.2014.10.032 |
| [14] | Kao TI, Chen PJ, Wang YH, et al. Bletinib ameliorates neutrophilic inflammation and lung injury by inhibiting Src family kinase phosphorylation and activity[J]. Br J Pharmacol, 2021, 178(20): 4069-4084. DOI:10.1111/bph.15597 |
| [15] | Day E, Waters B, Spiegel K, et al. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib[J]. Eur J Pharmacol, 2008, 599(1/2/3): 44-53. DOI:10.1016/j.ejphar.2008.10.014 |
| [16] | Botros L, Pronk MCA, Juschten J, et al. Bosutinib prevents vascular leakage by reducing focal adhesion turnover and reinforcing junctional integrity[J]. J Cell Sci, 2020, 133(9): jcs240077. DOI:10.1242/jcs.240077 |
| [17] | Rizzo AN, Sammani S, Esquinca AE, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2015, 309(11): L1294-L1304. DOI:10.1152/ajplung.00031.2015 |
| [18] | Hawez A, Ding ZY, Taha D, et al. C-Abl kinase regulates neutrophil extracellular trap formation and lung injury in abdominal sepsis[J]. Lab Invest, 2022, 102(3): 263-271. DOI:10.1038/s41374-021-00683-6 |
| [19] | Rizzo AN, Aman J, van Nieuw Amerongen GP, et al. Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome[J]. Arterioscler Thromb Vasc Biol, 2015, 35(5): 1071-1079. DOI:10.1161/ATVBAHA.115.305085.[PubMed |
| [20] | Do-Umehara HC, Chen C, Urich D, et al. Suppression of inflammation and acute lung injury by Miz1 via repression of C/EBP-Δ[J]. Nat Immunol, 2013, 14(5): 461-469. DOI:10.1038/ni.2566 |
| [21] | 刘雅茹, 李端阳, 杨红, 等. 博舒替尼对内毒素血症小鼠急性肺损伤的影响[J]. 中华麻醉学杂志, 2022, 42(11): 1370-1374. DOI:10.3760/cma.j.cn131073.20220606.01120 |
| [22] | Ulasov AV, Rosenkranz AA, Georgiev GP, et al. Nrf2/Keap1/ARE signaling: towards specific regulation[J]. Life Sci, 2022, 291: 120111. DOI:10.1016/j.lfs.2021.120111 |
| [23] | 武国艳, 温宇英, 白祥琰, 等. 硫辛酸通过Nrf2-ARE信号通路对百草枯中毒大鼠急性肺损伤保护作用的研究[J]. 中华急诊医学杂志, 2017, 26(7): 773-778. DOI:10.3760/cma.j.issn.1671-0282.2017.07.011 |
| [24] | Yin YY, Xu N, Qin T, et al. Astaxanthin provides antioxidant protection in LPS-induced dendritic cells for inflammatory control[J]. Mar Drugs, 2021, 19(10): 534. DOI:10.3390/md19100534 |
| [25] | 徐标, 李鸣, 王继武, 等. 盐酸纳美芬缺血后处理通过AMPK/Nrf2/HO-1途径减轻大鼠肺缺血-再灌注损伤的时效性研究[J]. 中华急诊医学杂志, 2023, 32(12): 1686-1692. DOI:10.3760/cma.j.issn.1671-0282.2023.12.019 |
| [26] | Maher J, Yamamoto M. The rise of antioxidant signaling: the evolution and hormetic actions of Nrf2[J]. Toxicol Appl Pharmacol, 2010, 244(1): 4-15. DOI:10.1016/j.taap.2010.01.011 |
| [27] | Bao LP, Li JS, Zha DQ, et al. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-κB pathways[J]. Int Immunopharmacol, 2018, 54: 245-253. DOI:10.1016/j.intimp.2017.11.021 |
| [28] | Zhao CZ, Xiao CS, Feng SQ, et al. Artemisitene Alters LPS-Induced Oxidative stress, inflammation and Ferroptosis in Liver Through Nrf2/HO-1 and NF-κB Pathway[J]. Front Pharmacol, 2023, 14: 1177542. DOI:10.3389/fphar.2023.1177542 |
 2024, Vol. 33
2024, Vol. 33




